Jeffrey Zhao
Northwestern University
Feinberg School of Medicine
Chicago
Carlos Galvez, MD
Department of Medicine
Northwestern University
Feinberg School of Medicine
Chicago
Jeffrey A. Sosman, MD
Robert H. Lurie Comprehensive Cancer Center
Northwestern University
Chicago
The State of Adjuvants in 2018
Recent advances in immunotherapy with antibody-based immune checkpoint blockade (ICB) and targeted kinase-directed small molecule therapies have transformed the adjuvant armamentarium for high-risk cutaneous melanoma. Using these agents following surgical intervention in the management of patients with American Joint Committee on Cancer (AJCC) stage III disease with lymph node involvement at high risk of relapse, oncologists have achieved five-year survival rates of 40 percent1 and decreased the risk of recurrence with disseminated disease.
Prior to 2015, options for adjuvant treatment following surgery were limited to interferon alfa-2b (Intron A®). High-dose IFNα-2b provided a minimal clinical benefit in high-risk patients and had an unfavorable adverse event profile. A comprehensive meta-analysis of 14 trials found modest effects of treatment, with a statistically significant improvement in disease-free survival in 10 of 17 comparisons to observation only and improved overall survival in only four of 14 comparisons (hazard ratios for disease recurrence and death of 0.82 and 0.89, respectively).2,3 High-dose IFNα-2b therapy was associated with toxicities such as fatigue, fever, myalgia and myelosuppression, which occur in a majority of patients.4
With the development and rapid approval of new adjuvant regimens in the last few years, the viability of initiating different therapies in high-risk stage III melanoma patients allows for more effective options for the physician. But it comes with added complexity due to the need to navigate a barrage of recent trial data. Additionally, based on results from the second Multicenter Selective Lymphadenectomy Trial (MSLT-II), completion lymph node dissection (CLND) is no longer recommended for patients with a single sentinel lymph node involvement.5 This should not impact the choice of adjuvant therapy, since CLND has proven to have no impact on overall survival.
The goal of this review is to summarize the exciting new advances in adjuvant therapy for stage III and stage IV (surgically resected) melanoma while also providing data-driven expert recommendations for practicing physicians.
Patient Risk Stratification
Risk stratification is an important factor in the decision to initiate adjuvant therapy for high-risk patients. Prognostic factors in the eighth edition AJCC clinical staging guidelines are used for stratifying risk in advanced melanoma, and tumor BRAF mutation status has a role in determining potential benefits and options for adjuvant therapy use.6 Indicators known to predict disease recurrence and dissemination include the extent of lymph node involvement, tumor depth, ulceration and presence of non-nodal locoregional metastases (any number of in-transit, satellite and/or microsatellite metastases that have not yet reached the regional nodal basin).7
Online calculators, including one developed by the AJCC Melanoma Database (available at melanomaprognosis.net for the AJCC seventh edition), allow for quick determination of risk of recurrence using information from large, validated patient datasets, but are still variable in their prediction.8 Given the predicted prognosis, subsequently the provider and patient must primarily weigh therapeutic efficacy with potential side effects, but also must factor in the financial cost of expanding the number of treatment regimens and the costs associated with managing therapy-related toxicities.9
Within AJCC (seventh edition) TNM-based clinical staging groups, adjuvant therapy studies with the highest level of evidence have focused on exploring recurrence-free and overall survival benefits in patients with stage III and resected stage IV disease.1,6 Stage III patients — those who have confirmed lymph node involvement but no distant metastases — represent the preponderance of the study population in recent trials. This focus on high-risk stage III disease is warranted, given the heterogeneous prognosis for patients within this group. Recent AJCC survival data suggest a 60 percent difference in five-year survival between lower-risk IIIA and higher-risk IIID subgroups.7 In contrast, there has been little focus on studying adjuvant therapy in stage II patients until now. Several older major trials (ECOG 1684, 1690, 1694) included only a limited number of stage IIC patients for randomization to high-dose IFNα-2b or observation. However, high-dose IFNα-2b is largely irrelevant today.1
Adjuvant Immune Checkpoint Blockade
There are currently two checkpoint inhibitors FDA-approved for use as adjuvant agents in advanced melanoma, with a third agent under FDA review and in line to be approved in the next few months. Several major studies have explored the efficacy of these unique agents as adjuvant therapies for high-risk stage III and resected stage IV disease.
Ipilimumab (Yervoy®), nivolumab (Opdivo®) and pembrolizumab (Keytruda®) ICB therapies inhibit key immune autoregulatory pathways normally involved in immune tolerance.10 Ipilimumab, a fully humanized IgG1 monoclonal antibody targeting the CTLA-4 molecule, was first approved by the FDA in 2011 for late-stage unresectable melanoma and later approved for adjuvant therapy in 2015. The human IgG4 anti-PD-1 inhibitor nivolumab was FDA-approved for advanced metastatic melanoma in late 2014 and then in the adjuvant setting in December 2017, and pembrolizumab will almost certainly be approved for a similar earlier-stage indication in the next few months. Mechanisms driving therapeutic efficacy with these agents can also lead to a wide spectrum of immune-related adverse events (irAEs) that can affect nearly every organ system including the skin, GI tract, endocrine organs, liver and other organs in a substantial number of patients.11
Clinical trial EORTC 18071 showed that ipilimumab increases recurrence-free survival and overall survival compared to placebo as an adjuvant agent for stages IIIA (LN >1 mm in size), IIIB and IIIC disease.12,13 Following this trial, CheckMate 238 directly compared nivolumab to ipilimumab in 906 patients with stages IIIB, IIIC (no stage IIIA) or resected stage IV melanoma.14 In this trial, the outcomes for the ipilimumab arm were similar to the previously reported EORTC 18071 trial of ipilimumab vs. placebo.14 Nivolumab had a significantly higher 24-month recurrence-free survival (63%; 95% CI, 66.1 to 74.5) in CheckMate 238 compared to ipilimumab (50%; 95% CI, 56.0 to 65.2) with a hazard ratio for disease recurrence or death of 0.66 (95% CI 0.54 to 0.81; P<0.0001).
Interestingly, this benefit appeared to be independent of tumor PD-L1 expression status. Not only did ipilimumab display an inferior clinical benefit, it also generated a threefold greater rate of grade 3 or 4 adverse events (45.9% vs 14.4%), with discontinuation of treatment in 43 percent of the ipilimumab group and 10 percent of the nivolumab group. Collectively, these data support use of nivolumab over ipilimumab in stages IIIB, IIIC and resected stage IV melanoma due to its superior clinical benefit and lower side effect profile. Overall survival benefit has not yet been demonstrated, but the number of deaths is still very small.
In the phase 3 KEYNOTE-054 study enrolling 1,019 patients with resected stage IIIA (LN>1 mm)–IIIC disease,15 adjuvant pembrolizumab (200 mg IV every three weeks) was associated with increased 12-month and 18-month recurrence-free survival (RFS) compared to placebo: a 12-month RFS rate of 75 percent for pembrolizumab compared to 61 percent for placebo and an 18-month RFS rate of 71 percent for pembrolizumab compared to 53 percent for placebo. RFS in the overall intention-to-treat (ITT) population and in the subgroup of patients with cancer that was positive for the PD-1 ligand (PD-L1) were the primary endpoints. In the ITT and PD-L1+ cohorts (as described above), the adjuvant pembrolizumab group also had significantly increased RFS compared to placebo, with a hazard ratio of 0.57 (CI 0.43 to 0.74; P<0.001) for ITT and 0.54 for PD-L1+ (CI, 0.43 to 0.74; P<0.001). Follow-up at 18 months revealed a significant RFS difference of 71 percent (CI, 66.8 to 75.4) for pembrolizumab vs. 53 percent (CI, 47.9 to 58.2) for placebo.
As in previous research, expression of the PD-L1 ligand did not predict therapeutic benefit, with a nonsignificant trend toward improved RFS for PD-L1- positive tumors. In this trial, 78 percent of patients in the pembrolizumab arm reported adverse events compared to 66 percent of placebo controls, with irAEs occurring in 37 percent of the treatment arm. However, more important, the total incidence of grade 3 adverse events for pembrolizumab (15 percent) was significantly lower than the reported rates for ipilimumab (46 percent) and on par with nivolumab (14 percent) in CheckMate 238.14 The most common grade 3 or 4 irAEs reported were colitis (2 percent), hypophysitis or hypopituitarism (0.6 percent) and type 1 diabetes mellitus (1.0 percent).
Taken together, these recent trials allow for stratification of adjuvant nivolumab, ipilimumab and pembrolizumab therapies by clinical benefit and tolerability. Nivolumab, FDA-approved as an adjuvant, emerges as a clear first-line agent for stage III disease given its increase in RFS endpoints compared to ipilimumab and its threefold reduction in severe adverse drug events compared to ipilimumab in CheckMate 238.
Pembrolizumab would be a viable alternative to nivolumab, with likely benefits over ipilimumab for adjuvant therapy, but is not yet approved for this indication. There are no published data comparing pembrolizumab against nivolumab or ipilimumab directly, although relative clinical benefit can be extrapolated from a trial of pembrolizumab against placebo and its shared target of action with nivolumab.15 An active phase 3 trial, SWOG 1404, is comparing outcomes for pembrolizumab versus ipilimumab or IFNα-2b, with the field awaiting results from this large study (NCT02506153). Also, a fully accrued trial, CheckMate-915, comparing nivolumab to the combination of ipilimumab and nivolumab will report its findings, but will likely require a few years before obtaining results.
Targeted Therapies
An alternative strategy for adjuvant therapy is the use of targeted therapies that act on intracellular signaling pathways. Targeted therapy agents are a broad drug class that includes small molecule inhibitors of kinases involved in mitogenic signaling pathways downstream of RAS, including BRAF and its downstream effectors MEK and then ERK.16,17,18 Inhibitors of BRAF such as dabrafenib (Tafinlar®), vemurafenib (Zelboraf®) and encorafenib (Braftovi®) have previously been approved for advanced BRAFV600-mutant melanoma and are respectively used in conjunction with the MEK inhibitors trametinib (Mekinist®), cobimetinib (Cotellic®) and binimetinib (Mektovi®). In addition to clinical grading of patients prior to treatment, these agents obviously require molecular testing of tumors for presence of BRAFV600-class mutations found in 40–50 percent of cutaneous melanomas.18
The FDA recently approved the targeted BRAF-MEK blockade combination therapy dabrafenib-trametinib for stage III melanoma patients with BRAFV600E– or BRAFV600K-positive tumors, based on the results of the COMBI-AD trial, which demonstrated significant increases in recurrence-free survival for stages IIIA (LN>1 mm) to stage IIIC patients at one to four-year time points with a median follow-up of 3.7 years (updated at ESMO 2018).19 Randomization to dabrafenib-trametinib resulted in 54 percent (CI 0.49-0.59) RFS at four years compared to 38 percent for placebo (hazard ratio 0.49; CI 0.40-0.59). Overall survival with a median follow-up of 2.8 years was also higher in the treatment group, with 86 percent surviving at three years compared to 77 percent for the placebo group of patients.
Combination dabrafenib-trametinib was associated with adverse events in 97 percent of 422 patients randomized to treatment compared to 88 percent randomized to placebo. The most common therapy-associated adverse events included pyrexia (63 percent), fatigue (47 percent) and nausea (40 percent). Notably, there were reported instances of new-onset malignancy including new melanoma in 11 patients (3 percent), either cutaneous squamous cell carcinoma or keratoacanthoma in eight patients (2 percent) and basal cell carcinoma in 19 (4 percent). More important, significant grade 3 and 4 toxicity were seen in 36 percent of patients on the combination and 10 percent on the placebo. Twenty-six percent of patients in the combination arm discontinued the trial permanently due to severe adverse events, while 38 percent experienced a dose reduction due to adverse events.
Vemurafenib has also been studied for adjuvant therapy, but it is not FDA-approved for this indication. The 2018 BRIM8, phase 3 trial reported data from cohorts of 498 stage IIC, IIIA and IIIB (cohort 1, n = 314) or fully resected stage IIIC (cohort 2, n = 184) BRAFV600-positive patients randomized to vemurafenib 960 mg or placebo for 52 weeks.20 Median study follow-up was 33.5 months in cohort 2 and 30.8 months in cohort 1.
In cohort 2, median disease-free survival was 23.1 months in the vemurafenib group versus 15.4 months in the placebo group, but the study did not reach its primary DFS endpoint. With cohort 2 not meeting its primary endpoint, the cohort 1 analysis was simply exploratory. Common adverse events reported were arthralgia, alopecia, dermatologic events such as rash, photosensitivity reactions, pruritus and hyperkeratosis. Additionally, the vemurafenib treatment group reported several new-onset cutaneous malignancies including squamous cell carcinoma, keratoacanthoma and basal cell carcinoma.
Given the impressive RFS benefit in COMBI-AD, combination dabrafenib-trametinib was FDA-approved and should be considered a first-line option for adjuvant therapy in patients with stage III BRAFV600E/K-expressing tumors. Estimated overall survival reached at three-year endpoints in the study of adjuvant vemurafenib is not significant as required in the protocol at this early time point. Additional data are needed to determine whether there is a role for vemurafenib in adjuvant therapy, but this is highly unlikely for the single agent alone.
Future Directions
Recently proposed trials include BRAF-MEK targeted agents combined with anti-PD-1 checkpoint blockade agents versus single-agent arms of either treatment alone. Furthermore, a phase 3 trial (KEYNOTE 716) of stages IIB and IIC patients comparing pembrolizumab versus placebo is under way, and accrual will likely be completed quickly.
An especially exciting area of research is the search for prognostic markers that could predict which patients will benefit from adjuvant therapy. In multiple trials, tumor PD-L1 expression has failed to correlate with the differences in RFS between adjuvant treatment and placebo groups.10 Potential blood markers that could be explored in future trials include the neutrophil-to-lymphocyte (N/L) ratio, which has been independently associated with mortality in stage II and III patients undergoing surgical resection,21,22,23 as validated in several retrospective analyses and a recent meta-analysis. The blood N/L ratio is not commonly used, but easily calculated in current clinical practice. Other assays being studied in advanced disease may ultimately be useful for those receiving adjuvant treatment.
Considerations for Clinical Practice
Given the current evidence, adjuvant therapy should be considered in patients with all AJCC stage IIIA (>1 mm LN), IIIB, IIIC, IIID and resected stage IV disease. Although the BRIM8 trial included a limited number of stage IIC patients, there are scant data to support current adjuvant therapy in high-risk lymph node-negative stage IIB or IIC disease. We recommend against adjuvant use in stages IIB, IIC and IIIA disease with LN metastases <1 mm (which are the majority of IIIA) due to the lack of supporting data and the potentially unfavorable risk-benefit profiles of starting therapy. Instead, we recommend enrollment into active clinical trials comparing an immune checkpoint inhibitor arm to placebo or other trials that are open to enrollment.
For stages IIIB, IIIC, IIID and resected stage IV patients with known BRAFV600E/K mutations, we cannot make a firm, data-supported recommendation of dabrafenib-trametinib or nivolumab over the other as first-line adjuvant therapy. The selection is not simple, due to the lack of long-term follow-up for the targeted combination or nivolumab, especially because of concern about a potential increase in late relapses in the dabrafenib-trametinib-treated cohort based on precedents in the stage IV setting. The selection is also complicated due to the severe immune-related toxicities that can occur in a small fraction of patients on the anti-PD-1, nivolumab or pembrolizumab therapies. Certainly, patients who are at high risk for immune-related toxicity, such as patients with active autoimmune disease or those who have undergone a prior allogeneic organ or bone marrow transplant, should receive targeted therapy.
The other cohort where treatment is not established is stage IIIA disease with small lymph node metastases (<1 mm diameter). (The eighth edition of the AJCC Staging Manual now classifies primary tumors that are >2 mm and involve sentinel lymph nodes as IIIB instead of IIIA.) Patients with this volume of disease have not been included in any of the recent trials and likely have a low risk for recurrence and death, while therapy may have a risk-benefit ratio that makes observation preferable. In the future, adjuvant therapy will lead to scenarios where anti-PD-1 or targeted therapy has been administered and the patient relapses. The question is who will benefit from retreatment in these instances, and how long will the disease-free period have to last to begin retreatment?
Given the promising data that have emerged for the new adjuvant therapies, further research into novel combinations of these agents as well as ongoing and future trials that directly compare targeted therapies to immunotherapy regimens will provide additional information on when best to use which agents in high-risk patients. 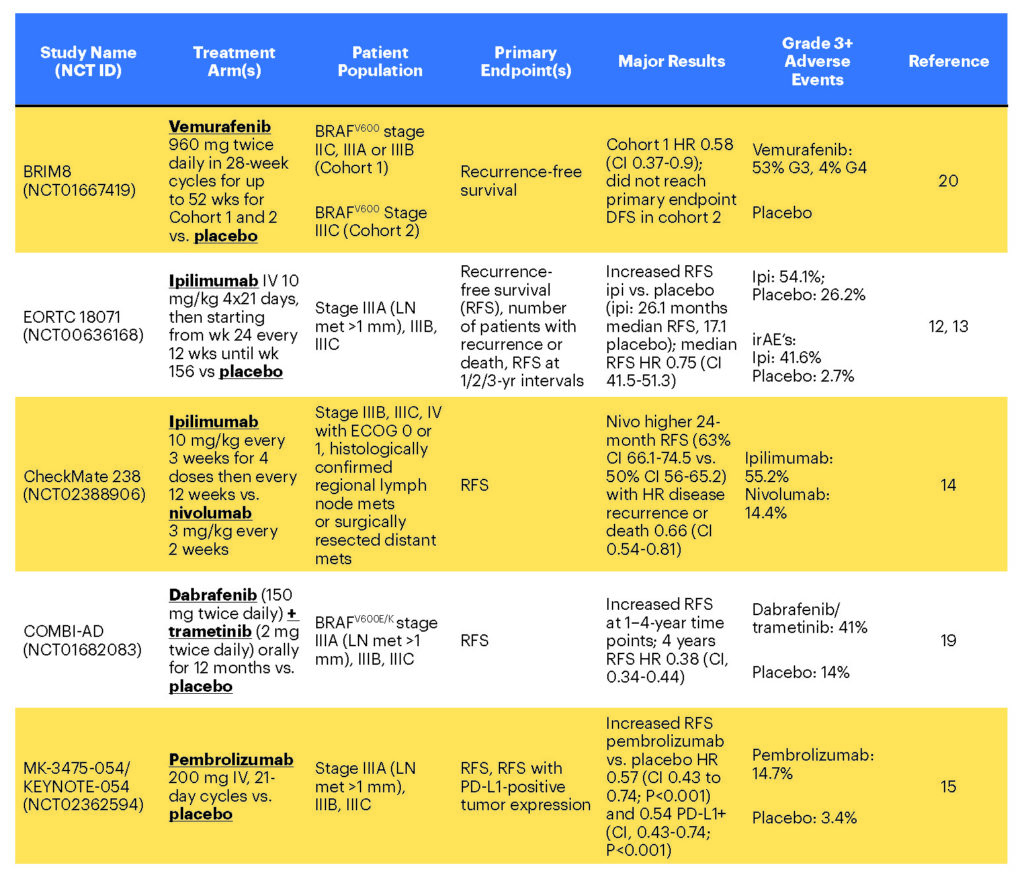
References
- Eggermont AM, Robert C, Ribas A. The new era of adjuvant therapies for melanoma. Nat Rev Clin Oncology 2018; 15(9):535-536. doi:10.1038/s41571-018-0048-5.
- Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. JNCI 2010; 102(7):493-501.
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14(1):7-17.
- Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose interferon of alfa-2b therapy. J Clin Oncol 2002; 20:3703.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. NEJM 2017; 376(23):2211-22.
- Warner AB, Postow MA. The brim of uncertainty in adjuvant treatment of melanoma. Lancet Oncol 2018; 19(4):436-7.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA: a cancer journal for clinicians 2017; 67(6):472-92.
- Zabor EC, Coit D, Gershenwald JE, et al. Variability in predictions from online tools: a demonstration using Internet-based melanoma predictors. Ann Surg Oncol 2018; 22:1-6.
- Kohn CG, Zeichner SB, Chen Q, et al. Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol 2017; 35(11):1194.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359(6382):1350-5.
- Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA 2018; 320(16):1702-3.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16(5):522-30.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. NEJM 2016; 375(19):1845-55.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. NEJM 2017; 377(19):1824-35.
- Eggermont AM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. NEJM 2018; 378(19):1789-801.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutation. NEJM 2012; 367: 18:1694-1703.
- Sullivan RJ, Infante JR, Janku F, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov 2018; 8(2):184-195. doi:10.1158/2159-8290.CD-17-1119.
- Davis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean? Cancer 2018; 124(17):3490-3499. doi:10.1002/cncr.31345. Epub 2018 Apr 17.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. NEJM 2017; 377(19):1813-23.
- Maio M, Lewis K, Demidov L, et al. Adjuvant vemurafenib in resected, BRAF V600 mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 2018; 19(4):510-20.
- Ding Y, Zhang S, Qiao J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: Evidence from a PRISMA-compliant meta-analysis. Medicine 2018; 97(30).
- Wade RG, Robinson AV, Lo MC, et al. Baseline neutrophil-lymphocyte and platelet-lymphocyte ratios as biomarkers of survival in cutaneous melanoma: a multicenter cohort study. Ann Surg Oncol 2018; 25(11):3341-9.
- Ma J, Kuzman J, Ray A, et al. Neutrophil-to-lymphocyte ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Scientific Reports 2018; 8(1):4044.
