Jedd D. Wolchok, MD, PhD
Memorial Sloan Kettering Cancer Center
Ludwig Institute for Cancer Research
and Weill Cornell Medical College
New York, NY
James Larkin, MD, PhD
Department of Medical Oncology
Royal Marsden Hospital
London, UK
Agents targeting immune checkpoint pathways have proven to be effective treatments for advanced melanoma. These pathways normally function to block both T-cell activation and T-cell function in peripheral tissues. Two well-defined and clinically validated targets for checkpoint blockade are the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) and PD-1 (programmed death-1) inhibitory pathways.1,2 Preclinical studies, and an evaluation of the immunologic effects of immune checkpoint blockade in human T cells and monocytes, demonstrated that the CTLA-4 and PD-1 pathways are nonredundant and have complementary roles in regulating different phases of T-cell activation.3,4 In vivo data from mouse models further demonstrated that blockade of both CTLA-4 and PD-1 induces synergistic immune-mediated antitumor activity.5,6 These observations supported clinical evaluation of combination therapy blocking both CTLA-4 and PD-1 in advanced melanoma. Consistent with these data, the combination of nivolumab (Opdivo®) and ipilimumab (Yervoy®) has proven to be an effective therapeutic strategy.
Ipilimumab, a monoclonal antibody that blocks the CTLA-4 receptor, was the first therapy to demonstrate an improvement in overall survival (OS) in a randomized, controlled, phase 3 trial of patients with advanced melanoma.7 A pooled analysis of data from 12 studies, including follow-up to 10 years in some patients, showed that ~20 percent of patients experienced long-term survival with ipilimumab monotherapy.8 In 2011, ipilimumab became the first immune checkpoint inhibitor to be approved for the treatment of unresectable or metastatic melanoma.
Nivolumab is a fully human monoclonal IgG4 antibody that binds PD-1 with high affinity and prevents interaction with its ligands, PD-L1 and PD-L2.1 As with ipilimumab, nivolumab monotherapy is approved for the treatment of unresectable or metastatic melanoma.
In 2016, the combination of nivolumab plus ipilimumab received regulatory approval in both the U.S. and the European Union for the treatment of unresectable or metastatic melanoma, regardless of BRAF mutation status. With its approval, the standard of first-line care for BRAF wild-type melanoma is now single agent anti-PD-1 therapy or the combination of nivolumab and ipilimumab. In BRAF-mutant melanoma, first-line options are now either the checkpoint blockade therapies or the targeted combination therapies, the latter combining the BRAF inhibitor dabrafenib (Tafinlar®) with the MEK inhibitor trametinib (Mekinist®) or the BRAF inhibitor vemurafenib (Zelboraf®) with the MEK inhibitor cobimetinib (Cotellic®). To date, no strong data have been reported for optimal sequencing of checkpoint inhibitor and BRAF-targeted therapy in BRAF-mutant melanoma. Thus, clinicians generally make recommendations on an individual basis, taking into account both tumor and patient characteristics.
Despite some significant adverse events (AEs) associated with the use of combination nivolumab-ipilimumab, clinical experience has proven the therapy’s effectiveness, and physicians can usually manage AEs using established treatment guidelines. Nonetheless, certain key questions remain unanswered regarding use of this combination therapy in the treatment of advanced melanoma.
Clinical Studies Evaluating Nivolumab-Ipilimumab
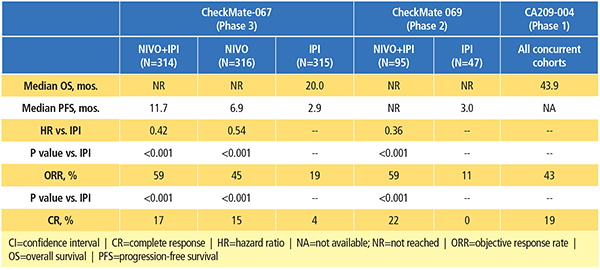
The combination of nivolumab and ipilimumab has been investigated in patients with advanced melanoma in phase 1, 2 and 3 clinical trials (Table 1).9-13 Data from the randomized clinical trials have demonstrated its superior efficacy compared to ipilimumab alone, albeit with a higher incidence of treatment-related AEs.11-13
Efficacy
CA209-004 was a phase 1, dose-escalation study to evaluate the safety of nivolumab combined with ipilimumab in patients with advanced melanoma.9,10 In four of the cohorts, patients received escalating doses of concurrent nivolumab plus ipilimumab every three weeks for four total doses, followed by nivolumab alone every three weeks for four total doses, then the combination every 12 weeks for a total of eight doses. An additional cohort of patients (cohort 8) received nivolumab 1 mg/kg and ipilimumab 3 mg/kg, every three weeks for a total of four doses, followed by nivolumab 3 mg/kg every two weeks for up to 48 weeks. In the end, this study established the dose and schedule used in cohort 8 as the regimen for further clinical evaluation.
A recent update of the CA209-004 study demonstrated that patients treated with the combination achieved high response rates and durable tumor responses, even in patients with poor prognostic factors at baseline (such as elevated serum lactate dehydrogenase [LDH] levels).10 Long-term follow-up demonstrated a two-year overall survival (OS) rate of 73 percent across all cohorts, with a median OS of 43.9 months, as well as encouraging survival rates for patients with baseline LDH levels higher than the upper limit of normal.
In the phase 2 study, CheckMate 069, patients with previously untreated advanced melanoma were randomized to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg once every three weeks for four doses followed by nivolumab monotherapy at 3 mg/kg once every two weeks, vs. ipilimumab monotherapy at 3 mg/kg.11 The primary endpoint of this study was objective response rate (ORR) among patients with BRAF V600 wild-type tumors. The confirmed ORR rate was 61 percent for the combination group vs. 11 percent in the ipilimumab monotherapy group (P<0.001).
Based on a recent analysis of all randomized patients (with and without a BRAF mutation) in CheckMate 069, the two-year OS rate was numerically higher (63.8 percent) with the combination than with ipilimumab alone (53.6 percent), although the difference was not statistically significant (Figure 1).12 Median OS had not yet been reached in either group, showing that follow-up beyond two years may be required to realize the full survival potential of combination therapy. Notably, 70 percent of patients in the ipilimumab-alone group received any subsequent therapy, compared with 35 percent in the combination group. The subsequent therapy included anti-PD-1 therapy in 62 percent and 18 percent of patients, respectively.
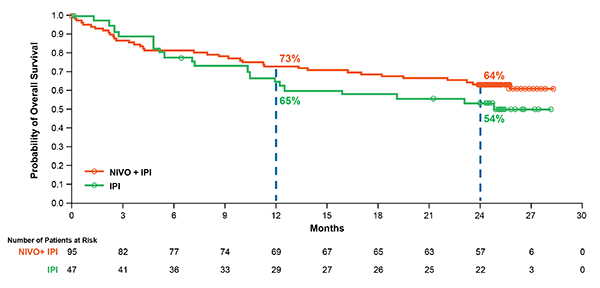 Figure 1. Overall Survival at Two Years of Follow-up in Patients Who Received Nivolumab–Ipilimumab Combination Therapy or Ipilimumab Alone in a Phase II Trial. At a median follow-up of 24.5 months in the CheckMate 069 trial, two-year OS rates were 63.8 percent and 53.6 percent, respectively, in all randomized patients who received nivolumab plus ipilimumab or ipilimumab-alone, with median OS not reached in either group (hazard ratio, 0.74; 95% CI, 0.43–1.26). In the ipilimumab-alone group, 70 percent of patients received any subsequent therapy upon disease progression. Reprinted with permission from Elsevier.
Figure 1. Overall Survival at Two Years of Follow-up in Patients Who Received Nivolumab–Ipilimumab Combination Therapy or Ipilimumab Alone in a Phase II Trial. At a median follow-up of 24.5 months in the CheckMate 069 trial, two-year OS rates were 63.8 percent and 53.6 percent, respectively, in all randomized patients who received nivolumab plus ipilimumab or ipilimumab-alone, with median OS not reached in either group (hazard ratio, 0.74; 95% CI, 0.43–1.26). In the ipilimumab-alone group, 70 percent of patients received any subsequent therapy upon disease progression. Reprinted with permission from Elsevier.The use of anti-PD-1 treatment as the subsequent therapy likely explains, in part, why the two-year OS rate with ipilimumab in this study was much higher than reported in a phase 3 study of ipilimumab in previously treated advanced melanoma (25.3 percent),14 which was conducted prior to the regulatory approvals of anti-PD-1 agents. In contrast, progression-free survival (PFS) rates in CheckMate 069 were significantly higher in the combination group than in the ipilimumab group.12 While median PFS was 3.0 months in the ipilimumab group, it was not reached in the combination group, and two-year PFS rates were 12.0 percent and 51.3 percent, respectively. (P<0.0001). To date, this analysis represents the longest survival follow-up of patients who received the combination of nivolumab and ipilimumab in a randomized, controlled trial.
CheckMate 067 was the first phase 3 trial designed to evaluate the combination of nivolumab and ipilimumab in patients with advanced melanoma.15 In this study, previously untreated patients were randomized to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every three weeks for four doses, followed by nivolumab 3 mg/kg every two weeks, vs. single-agent nivolumab 3 mg/kg every two weeks, vs. single-agent ipilimumab 3 mg/kg every three weeks for four doses. Patients were stratified by tumor PD-L1 expression and by BRAF mutation status. Co-primary endpoints were PFS and OS. The primary analysis of PFS demonstrated a significant improvement with the combination (11.5 months) and nivolumab alone (6.9 months) compared with ipilimumab alone (2.9 months). ORR was also significantly higher with the combination (57.6 percent) and nivolumab alone (43.7 percent) compared with ipilimumab alone (19.0 percent).
Recently, at a minimum follow-up of 28 months, investigators reported the first OS results for this study.16 A significant improvement in OS was demonstrated with the combination of nivolumab and ipilimumab or nivolumab alone compared with ipilimumab; two-year OS rates were 64 percent for the combination, 59 percent for nivolumab and 45 percent for ipilimumab.16
In analyses of predefined subgroups, the combination or nivolumab alone showed numerically higher ORR and longer PFS and OS than ipilimumab alone, even in patients with elevated LDH and M1c disease.15,16 At a minimum follow-up of 28 months, PFS and ORR results were similar to those of the primary analysis.16 Median duration of response had not yet been reached with the combination, vs. 31.1 months for nivolumab and 18.2 months for ipilimumab.16 While the study was not designed to formally compare the combination with nivolumab alone, the results of descriptive analyses showed that the combination resulted in numerically higher ORR and longer PFS as well as OS than nivolumab alone.16 Among high PD-L1 expressors, an OS and PFS benefit relative to ipilimumab was observed for both the combination and nivolumab alone, and was similar in both nivolumab-containing groups.15,16 However, nivolumab plus ipilimumab resulted in clinically meaningful improvements in ORR regardless of PD-L1 expression level.15
Safety Profile
The types of treatment-related AEs reported for the combination of nivolumab and ipilimumab are consistent with what has been previously reported for each agent alone. However, investigators have reported a higher frequency of treatment-related grade 3–4 AEs with the combination compared with either agent alone.11-13,16 Based on the most recent updates from phase 2 and 3 trials, grade 3–4 treatment-related AEs occurred in 54 to 58.5 percent, 20.8 percent and 20 to 28 percent of patients who received nivolumab plus ipilimumab, nivolumab monotherapy and ipilimumab monotherapy, respectively (Table 2).12,16 The most common AEs associated with nivolumab and ipilimumab treatment are select AEs (those with an immunologic etiology), and they affect diverse organ systems including the skin, gastrointestinal (GI) tract, endocrine system, liver, lungs, kidneys and heart muscle. In general, compared with nivolumab or ipilimumab monotherapy, combination therapy results in a higher incidence of GI, hepatic and endocrine select AEs.11-13,16 Treatment-related AEs led to discontinuation of therapy in 36 to 39.6 percent, 11.5 percent, and 9 to 16.1 percent of patients who received the combination, nivolumab alone and ipilimumab alone, respectively (Table 2).12,16
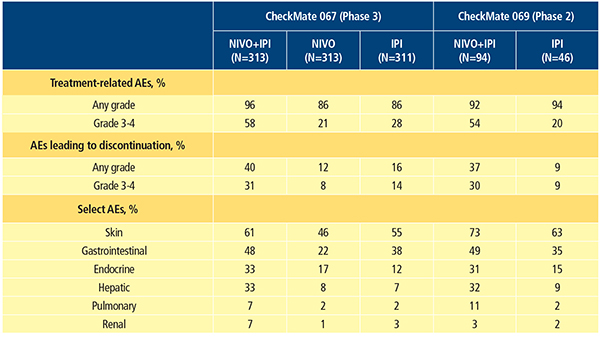
There are now established guidelines for management of nivolumab- and ipilimumab-associated select AEs. These guidelines include the use of immune-modulatory agents, most commonly systemic corticosteroids.11,17 Consistent with the higher incidence of select AEs, immune-modulatory agents were used in 83 to 89 percent of patients treated with the combination, 56 to 59 percent treated with ipilimumab monotherapy and 47 percent treated with nivolumab monotherapy in clinical trials.11,13 Even though a higher incidence of select AEs (including skin, gastrointestinal, hepatic, pulmonary and renal select AEs) was observed in the combination group, resolution rates for select AEs of grade 3–4 were between 85 percent and 100 percent, using standard treatment algorithms.11,13 However, AEs affecting the endocrine system were a notable exception. Many endocrine select AEs were considered unresolved due to the continuing need for hormone replacement.
Efficacy in Patients Who Discontinued Treatment Due to Toxicity
The high discontinuation rates due to toxicity with the combination regimen may engender concerns that patients who discontinue treatment early will have reduced clinical benefit. In the CheckMate 069 study, the researchers investigated the impact of discontinuation due to toxicity on patient responses and outcomes. In this analysis, similar response rates were observed in patients who did or did not discontinue the combination due to toxicity.18 Notably, in the 35 patients who discontinued due to treatment-related AEs, OS was similar to that of all randomized patients (one-year OS rate of 83 percent vs. 73 percent and two-year OS rate of 71 percent vs. 64 percent, respectively). Median duration of response was not reached in either group, with ongoing responses seen in 80 percent of all randomized patients who received the combination and in 74 percent of patients who discontinued treatment.
In a post hoc retrospective analysis of data from the CheckMate 067 trial, PFS and ORR were significantly greater in patients who discontinued treatment due to AEs compared with those who did not discontinue due to AEs.19 Despite reduced exposure to nivolumab and ipilimumab, these data suggest that patients who need to discontinue treatment due to an AE still benefit from combination therapy.
Remaining Challenges and Unanswered Questions
While there is considerable evidence for the efficacy and safety of nivolumab in combination with ipilimumab, a number of unanswered questions and challenges remain. Ongoing and future studies will aim to address them. For example:
Which patients are most likely to benefit from combination therapy?
– There is currently no validated biomarker to assist in patient selection.
– Investigators have consistently observed high objective response rates with combination therapy regardless of tumor PD-L1 expression, and the role of tumor PD-L1 expression as a biomarker for the efficacy of nivolumab plus ipilimumab remains unclear.
What are the long-term survival outcomes with combination therapy vs. anti-PD-1 monotherapy?
– Continued follow-up of the patients from key clinical studies will be required to determine long-term survival outcomes with combination therapy vs. anti-PD-1 monotherapy.
What is the optimal sequencing of therapies such as immune checkpoint inhibitors and targeted BRAF/MEK inhibitors to provide the greatest benefit to patients?
What is the optimal dose of each agent within the combination regimen?
– For one thing, the role of nivolumab maintenance needs to be defined.
Should patients receive treatment beyond progression with combination therapy, or switch to another therapy?
How can we identify patients at risk from rare but serious or fatal adverse events, such as
myocarditis?
In general, we still have much to learn about efficacy and safety. We need to attain a better understanding of AEs with long-term exposure and of the feasibility of rechallenge after resolution of the AE. The data for melanoma metastatic to the brain is still limited, and efficacy and safety in other disease settings remain to be determined, e.g., in settings of adjuvant use, autoimmune disease and in patients who are already receiving significant doses of immunosuppressive agents.
Summary and Future Implications
Combination therapy with immune checkpoint inhibitors is an effective treatment option for patients with advanced melanoma, including those with a BRAF mutation and those with poor prognostic factors. The combination of nivolumab and ipilimumab has demonstrated robust antitumor activity, and response data suggest that the combination may be superior to either agent alone.
Although the combination results in greater toxicity compared with each agent alone, the majority of treatment-related AEs resolve with established management guidelines. Available evidence suggests that patients who discontinue treatment due to AEs may still derive benefits from the combination regimen comparable to patients who remain on treatment. The optimal dosing of anti-PD-1 agents in combination with ipilimumab is currently being evaluated in clinical trials. A randomized, double-blind, phase 3b/4 study, CheckMate 511 (NCT02714218), is currently evaluating two different dose combinations of ipilimumab plus nivolumab, followed by nivolumab monotherapy, in patients with untreated advanced melanoma: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg vs. nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.
In addition, a phase 1/2 trial, KEYNOTE-029 (NCT02089685), is evaluating the efficacy of pembrolizumab (another monoclonal antibody targeting PD-1) at 2 mg every three weeks in combination with ipilimumab 1 mg/kg every three weeks.20 While some questions remain unanswered, it is clear that combination therapy has changed the treatment landscape for advanced melanoma and will continue to be an active area of investigation.
References
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33:1974-82.
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity 2016; 44:1069-78.
- Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015; 194:950-9.
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520:373-77.
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010; 107:4275-80.
- Selby M, Engelhardt J, Lu L-S, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol 2013; 31 (suppl; abstr 3061).
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33:1889-94.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33.
- Sznol M, Callahan MK, Kluger H, et al. Updated survival, response and safety data in a phase 1 dose-finding study (CA209-004) of concurrent nivolumab (NIVO) and ipilimumab (IPI) in advanced melanoma. Presented at the Society for Melanoma Research (SMR) 2015 International Congress, November 18–21, 2015, San Francisco, CA, USA.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372:2006-17.
- Hodi FS, Chesney J, Pavlick AC, et al. Two-year overall survival rates from a randomised phase 2 trial evaluating the combination of nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma. Lancet Oncol 2016; 17:1558-68.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23-34.
- McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S; MDX010-20 Investigators. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol 2013; 24:2694-8.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Efficacy and safety in key patient subgroups of nivolumab alone or combined with ipilimumab versus ipilimumab alone in treatment-naïve patients with advanced melanoma (CheckMate 067). Presented at the European Cancer Congress (ECC) 2015 Meeting, September 25–29, 2015. Vienna, Austria.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate 067). Presented at the American Association for Cancer Research Annual Meeting, April 1–5, 2017, Washington, DC, USA.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16:375-84.
- Hodi FS, Postow MA, Chesney J, et al. Overall survival in patients with advanced melanoma (MEL) who discontinued treatment with nivolumab (NIVO) plus ipilimumab (IPI) due to toxicity in a phase II trial (CheckMate 069). Presented at the American Society of Oncology 2016 Annual meeting, June 3–7, 2016. Chicago, IL, USA.
- Schadendorf D, Larkin J, Postow M, et al. Efficacy and safety outcomes in patients with advanced melanoma (MEL) who discontinued treatment with nivolumab (NIVO) plus ipilimumab (IPI) due to toxicity. Presented at the 16th World Congress on Cancers of the Skin/12th Congress of the European Association of Dermato-Oncology, August 31–September 3, 2016, Vienna, Austria.
- Long GV, Atkinson V, Cebon JS, et al. Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: Results of the KEYNOTE-029 expansion cohort. Presented at the American Society of Clinical Oncology 2016 Annual meeting, June 3–7, 2016. Chicago, IL, USA.

