Mark B. Faries, MD
Codirector, Melanoma Program
Head, Surgical Oncology Division
The Angeles Clinic and Research Institute
Surgical Director, Experimental Therapeutics
Cedars Sinai Medical Center
The initial surgical treatment of patients with melanoma has been a subject of discussion, research and controversy throughout much of the history of the disease. Over that time, we have learned a great deal about melanoma biology, and our treatments have become more personalized, accurate and well-tolerated. The recent publication of data from the second Multicenter Selective Lymphadenectomy Trial (MSLT-II) has refined our understanding and answered important questions about management.1 Here, we review the results of the trial to date and put them in context.
MSLT-II
Rationale and Study Design
Sentinel lymph node biopsy (SLNB) was initially devised as a tissue-sparing means of selecting patients to undergo complete lymph node dissection. Until then, patients with intermediate- or high-risk, clinically localized melanomas either had to rely on watching and waiting to see if nodal metastases became clinically apparent, or had to undergo complete elective lymph node dissection without knowing whether any of their nodes contained metastases. In most cases, nodes were negative. While this was good news for the prognosis of these patients, they had to pay the full morbidity cost of dissection to obtain that information. After Morton and Cochran established the SLNB technique in the early 1990s, clinically node-negative patients required only a minimally invasive lymph node biopsy, and only patients with a positive SLNB reflecting regional tumor spread were advised to have complete dissection. Thus, most patients could avoid the more extensive surgery.2
As experience grew with SLNB, it became apparent that in the vast majority of cases, patients with SLN metastases had no other involvement discovered when completion lymph node dissections (CLND) were subsequently performed.3 In most studies, 80 to 90 percent of patients had negative CLND pathology. It also became clear that patients with non-SLN involvement (involvement of non-sentinel nodes) had a poorer prognosis. Multiple series have found this adverse effect from non-SLN metastasis, demonstrating a qualitative difference between SLN and non-SLN metastasis.4 Patients with tumor penetration beyond the first echelon (sentinel) nodes in the regional basin have prognoses similar to patients who present with clinically palpable nodal metastases. As a result, clinical equipoise existed regarding the value of CLND compared with close observation, and the MSLT-II trial was designed to compare these options.
The trial consisted of two phases. In the first, screening phase, patients were enrolled prior to SLN biopsy. Screening patients underwent preoperative ultrasound of draining nodal basins, allowing evaluation of ultrasound accuracy in that setting. If the SLN was negative by standard pathology, it was submitted for evaluation by a multi-marker quantitative RT-PCR assay looking for molecular indications of melanoma in the nodes.5 Patients who were positive by either standard pathology or molecular evaluation were eligible for the second, randomization phase. In that phase, patients with SLN metastases were assigned to either standard CLND or observation including follow-up with nodal ultrasound. Those who developed subsequent nodal recurrence underwent delayed complete node dissection.
Trial Results
With more than 60 participating centers, the trial achieved complete accrual (n=1939). At the third interim analysis, the Data Safety Monitoring Board determined there was no reasonable possibility of a significant difference in melanoma-specific survival for either arm of the trial and recommended that the primary endpoint data be released. The trial did demonstrate a significant difference in disease-free survival. Examination of the types of recurrence showed that the effect on disease-free survival derived from an impact on regional nodal disease control, with a 70 percent reduction in regional recurrence after immediate CLND. In contrast, there was no significant impact on distant metastasis-free survival.
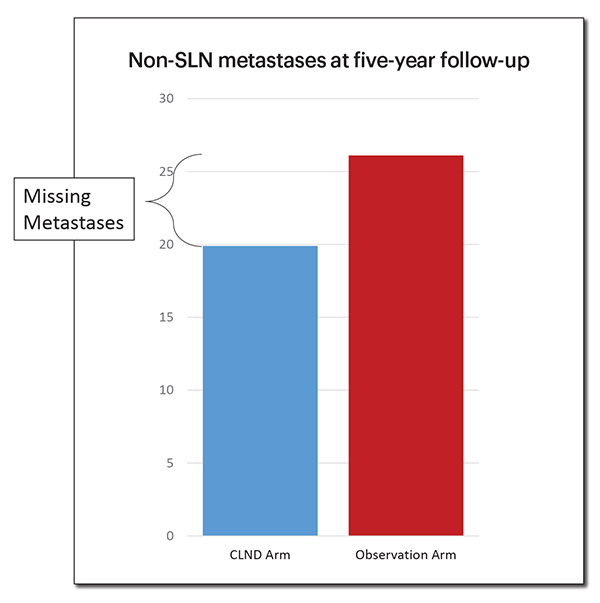
An additional interesting observation involved the cumulative rate of detected non-SLN metastases in the two arms of the trial. (Figure 1) The rate of non-SLN metastasis (as measured by in-basin nodal recurrence) was significantly higher in the observation arm compared with the rate in the CLND arm (which was the sum of the detected non-SLN metastases on CLND and in-basin recurrence). The increase of about 8 percent in the observation arm is likely due to occult metastases that were not detected by standard pathology within the CLND specimen and is similar to the percentage detected when those nodes have been subjected to immunohistochemical evaluation. While the rate of non-SLN metastasis found on CLND was 11 percent, the five-year actuarial rate of in-basin recurrence in the observation arm was 26 percent. This may be important for patients to consider if they elect observation. It is also important to bear in mind when developing algorithms for predicting non-SLN metastases, since standard methods using CLND specimens may miss close to half of all positive non-SLN.
Context
Origins: Lymph nodes are the obvious path of metastatic disease
The role of lymph nodes and nodal metastases in progression and treatment of melanoma has been a subject of controversy for well over a century. Where do the current clinical trial data fit within that larger issue of tumor biology? The question was initially raised by Herbert Snow, MD, a surgeon at the London Cancer Hospital, a forerunner of the current Royal Marsden.6 In 1892, Dr. Snow proposed in The Lancet what he called “anticipatory gland excision,” a forerunner of elective lymph node dissection. The operation, he said, was “a simple common-sense measure, adding nothing to the gravity of a surgical operation [the primary tumor removal], while most materially enhancing its efficacy.” Others in the surgical community challenged most aspects of Dr. Snow’s assertion, and the controversy was born.
Dr. Snow’s speculation on the role of lymph nodes was intuitive. In the majority of solid tumors, metastases frequently appear first, and often exclusively, in regional lymph nodes, and the presence of tumor in those nodes is generally the most powerful prognostic variable in initial staging. It is natural to assume that lymph nodes act as physical filters, trapping tumor cells for some period of time. In addition, it is clear from randomized trials that, in melanoma, if lymph node metastases are allowed to remain in place, they will grow and spread to other nodes within the same basin. In the MSLT-I trial, which randomized patients to wide excision alone or wide excision with SLN biopsy, observation-only patients developed twice the number of involved nodes, on average, compared with those who had immediate surgery.7
However, experimental evidence suggested that the presumed role of regional nodes as physical filters for tumor cells was not correct. Bernard and Edwin Fisher, MD, injected tumor cell suspensions into the afferent lymphatics or footpads of rabbits,8 then examined the effluent lymph exiting the popliteal lymph nodes, finding that a large proportion of the injected cells passed through the nodes immediately. They hypothesized that cancer spread systemically early in the course of the disease, so nodal interventions were no more than markers of a metastatic phenotype.
Eventually, in a series of randomized clinical trials, melanoma patients either underwent elective lymph node dissection, or were observed and underwent dissection only if they developed recurrence in the basin. These trials included two World Health Organization (WHO) studies (#1 and #14), conducted in Italy, a smaller trial at the Mayo Clinic and the Intergroup Study performed by the North American cooperative groups.9-12 These trials did not show a statistically significant survival advantage with early, elective dissection for melanoma patients as a whole.
The results parallel those of studies examining nodal surgery in multiple other tumor types. In breast cancer, the question was evaluated early on in the National Surgical Adjuvant Breast Program (NSABP) B-04 trial comparing radical mastectomy with node dissection against mastectomy alone or mastectomy with nodal irradiation.13 It found no survival advantage to early nodal surgery. In esophageal, lung and gastric cancer trials, more extensive nodal dissections did not lead to a statistically significant survival advantage compared with less extensive surgery.14-16
Mounting evidence: Why no benefit?
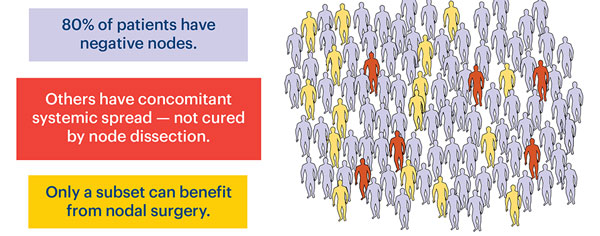
How can these negative clinical trial results be explained, given the intuitive observations of tumor behavior? There are several explanations for the disconnect, including statistical considerations, particularly a lack of statistical power in many of the trials. Additionally, in some cases, mortality associated with more extensive surgery counteracted a likely impact on the oncologic course of patients. Finally, biological differences among tumors affect their metastatic potential, and these differences make it likely that, while early nodal intervention offers benefits, only some subgroups of patients will reap those benefits.
Statistical power is a problem in many clinical studies — particularly randomized surgical trials. In many trials evaluating nodal interventions, the differences in results reasonably expected to be detectable were large. These differences could exist, and even be seen in the trial, while not leading to a significant p value. In all the elective lymph node dissection trials in melanoma, the groups undergoing early nodal treatment had numerically better survival than those who were observed, but in none of the trials did that difference achieve statistical significance. The reason is partly that the majority of patients presenting with a new diagnosis of primary cutaneous melanoma do not have nodal metastases. (Figure 2) Thus, most of the patients being treated in these studies could not derive a survival benefit from having nodes removed. Despite that design handicap, the trials showed broad consistency in suggesting a benefit. A meta-analysis of ELND trials in melanoma found a hazard ratio of 0.86 in the direction of a reduced risk of melanoma death with ELND.17 This number is quite similar to the hazard ratio of 0.89 seen in the MSLT-I trial.18 While the accompanying nonsignificant p values indicate that the results do not prove a benefit, they certainly do not show the two treatments to be equal.
In studies of other cancer types, such as the Dutch gastric cancer study comparing D1 (limited) to D2 (extended) lymph node dissections, the issue of operative mortality was also a problem. That study showed no difference in overall survival related to the more extensive dissection. However, that D2 group also experienced a 6 percent greater operative mortality. When that confounding factor was taken into account by examining gastric cancer-specific mortality, there was a significant benefit, which increased with time, for the D2 group.14 So the biological role of lymph nodes was supported, though without a practical survival benefit to patients.
Lymph nodes are probably not just markers of a metastatic phenotype
The initial assumption underlying the clinical utility of nodal surgery was that nodes acted as physical filters removing tumor cells from lymph fluid. As noted above, that filter hypothesis was experimentally disproven. In fact, other biological evidence suggests that regional nodes can function as incubators for tumor cells, facilitating their spread and protecting them from destruction. Multiple studies now show that the tumor-draining regional nodes are prepared to receive metastases through lymphangiogenesis.19,20 These changes, together with the presence of open-ended, low-shear lymphatic vessels, mean that tumor cells (which might not have developed the ability to invade blood vessels, survive the turbulence of the bloodstream or extravasate) are able to navigate to regional lymph nodes. Those same nodes also demonstrate features of diminished immunologic capacity.21,22 SLN that receive direct drainage from the tumor site have been found to have a decreased nodal area occupied by dendritic cells. In addition, those dendritic cells are characterized by markedly decreased length of dendritic processes, so the antigen detection network of normal nodes is compromised in tumor-draining nodes. These features indicate that the node may be an area where tumor cells can persist, expand and evolve after the primary tumor has been removed. So, physical filtration by the nodes is not necessary for the nodes to have clinical significance.
However, in some tumors, the ability to metastasize to distant sites may be present at the time of diagnosis. In those cases, the incubator function would no longer be relevant, and clinical benefit from early nodal surgery would be unlikely. There is good evidence of this phenomenon in melanoma. In both the ELND trials and in MSLT-I, some groups had fairly consistent benefit, and others consistently showed no benefit. Tumor thickness was one principal determinant of survival improvement or lack of improvement with early surgery. (Figure 3)
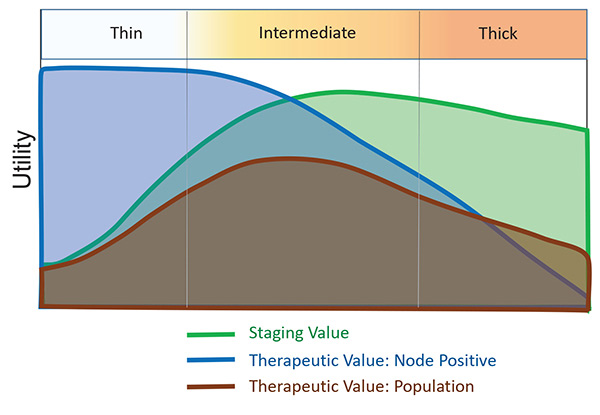
Retrospective series used as the basis for planning some of the ELND trials hypothesized a “sweet spot” in the thickness spectrum in which clinical benefit would be most likely to be found.23,24 For patients with thin melanomas, so few had nodal metastases that no study would ever find a statistical difference in outcome. For patients with thick melanomas, most patients with nodal metastases already had distant disease at the time of diagnosis, and thus also could not derive a survival benefit. It was only in the intermediate thickness range that the proportion of patients with metastases limited to regional nodes was high enough to find an effect of early treatment.
Some trials found that hypothesis to be correct. The WHO #14 trial showed a statistically significant improvement in survival for patients in the dissection arm if their primary tumor was 1.5 to 4 mm in thickness.25 Patients with thicker tumors had no indication of benefit. Similarly, in the Intergroup study, patients with tumors between 1 and 2 mm had significant benefit, while those with thicker tumors did not.26 Finally, in MSLT-I, when examining the node-positive patients in one arm of the study (who had either positive SLN or clinical nodal recurrence), there was a marked improvement in melanoma-specific survival (HR 0.56, p=0.006) for intermediate-thickness tumors (1.2 mm to 3.5 mm), but no improvement for those with thicker primaries.18 This consistency provides strong support for the therapeutic effect of early nodal treatment for intermediate-thickness melanomas while suggesting only a staging value in patients with thick tumors.
Treatment of lymph nodes for those with thin melanomas is particularly controversial. These tumors represent the most common thickness for newly diagnosed patients, and while nodal metastases in this group are infrequent, the preponderance of this thickness means that, in absolute numbers, thin melanomas are an important source of nodal metastases. The presence of SLN metastases for patients with thin melanomas does appear to indicate a worse prognosis compared with those who are node-negative, though their outcomes are favorable compared with patients who have metastases from thicker melanomas.27 In contrast, patients who develop clinically apparent nodal metastases from thin primary melanomas exhibit poor outcomes, similar to those who have metastases from thicker primaries.28 Hypothetically, the benefit of early removal of nodal metastases for those with thin melanoma may exceed that of intermediate or thick primary patients, since the odds are lower that the disease will have already spread to distant body sites. If this is true, though, it is difficult to act on, since performing SLN biopsy in all cases of thin melanoma would not be cost-effective. Current methods of determining which thin melanomas result in nodal metastases are not optimal, and further research will be important.
This concept is controversial. Some investigators do not believe that the tumor cells identified in SLN are biologically the same as clinical nodal recurrences presenting in patients who did not undergo initial nodal staging. However, the percentage of patients who have either positive SLN or clinical recurrence appears to be approximately the same when adjusted for tumor thickness. The magnitude of the effect on survival of early nodal treatment and the plausibility of this from a biological standpoint make it at least an intriguing hypothesis, though testing it may prove difficult.29 At minimum, it suggests that greater efforts are warranted to determine which patients with thin melanomas are likely to harbor nodal metastases.
Over all, MSLT-II shows that careful observation is a reasonable alternative to CLND for patients with SLN metastases. It appears that any survival benefit derived from early nodal surgery comes from the SLN biopsy and not the removal of non-SLN. CLND remains an option, but one that fewer patients will select.
Clinical Implications of MSLT-II
With the recent publication of the Multidisciplinary Selective Lymphadenectomy Trial II added to other available data on sentinel lymph node biopsy (SLNB) and completion lymph node dissection (CLND), it is important to reevaluate the guidelines for the surgical treatment of patients newly diagnosed with a clinically localized melanoma. Two key questions apply:
Is there still a role for completion lymph node dissection?
Although MSLT-II does not show a survival advantage for CLND, the technique does have potential value that patients should have the opportunity to consider. The pathologic status of non-SLN has independent and clinically meaningful prognostic significance. To date, this information cannot be reproduced using combinations of any other available indicators. For patients on the fence about whether to pursue adjuvant therapy, this information may tip the scales. In addition, completing the node dissection markedly reduces the risk of regional recurrence, with an accompanying smaller reduction in overall recurrences. Since even treatable nodal recurrences can be traumatic to patients, avoiding them may be desirable. Finally, the available data from both MSLT-II and the DeCOG-SLT1 trial are limited with regard to patients who have higher-volume nodal metastases. Such patients would be more likely to have non-SLN metastases but comprised only about one third of the trial population. It should be noted, however, that there was no trend toward increased benefit in that subgroup of the study.
Initial observation for those with positive SLNBs, conforming to the trial protocol, appears to be safe (does not seem to decrease overall survival). The relative contribution of ultrasound in that follow-up setting is yet to be fully understood and will require further study. However, the trial provides reassurance of the safety of observation only in the setting of close follow-up. Patients who cannot be reliably monitored, ideally with the use of high quality ultrasound included, would probably be better served with immediate CLND.
Does MSLT-II alter the rationale for SLN biopsy?
The results of MSLT-II do not diminish the rationale for SLN biopsy. In fact, since the minimally invasive procedure can now be performed without mandatory progression to completion in the setting of a positive SLN, the impetus to perform SLN staging should actually be increased. For patients with intermediate-thickness melanomas and nodal metastases, it appears that any survival benefit is derived from the SLN procedure. In patients with thick melanomas, the procedure provides the most important staging information. It also provides significant regional disease clearance and control, since in both intermediate thickness and thick melanomas, the SLN biopsy removes all regional disease in the majority of patients. For patients with regional nodal metastases, the best way to avoid complete lymph node dissection is to have an SLNB done.
Additional research is needed. Ideally, more data will allow us to better predict which patients have non-SLN metastases (or conversely, which patients certainly do not have such metastases). This would enable better counseling of patients and better selection of candidates for immediate completion. Thin melanomas also deserve additional attention. Better methods to identify the thin melanomas that are at increased risk for regional metastases may be among the most important things yet to be accomplished to enable the most rational, least morbid and most effective surgical treatment for this disease.
- Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicenter, randomized, phase 3 trial. Leiter U, Stadler R, Mauch C, et al. The Lancet 2016; 17:6:757-767.
References
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med 2017; 376:2211-22.
- Morton D, Wen D, Wong J, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127:392-9.
- Cochran AJ, Roberts A, Wen DR, et al. Optimized assessment of sentinel lymph nodes for metastatic melanoma: implications for regional surgery and overall treatment planning. Ann Surg Oncol 2004; 11:156S-161S.
- Leung AM, Morton DL, Ozao-Choy J, et al. Staging of regional lymph nodes in melanoma: a case for including nonsentinel lymph node positivity in the American Joint Committee on Cancer staging system. JAMA Surg 2013; 148:879-84.
- Nicholl MB, Elashoff D, Takeuchi H, Morton DL, Hoon DS. Molecular upstaging based on paraffin-embedded sentinel lymph nodes: ten-year follow-up confirms prognostic utility in melanoma patients. Ann Surg 2011; 253:116-22.
- Neuhaus SJ, Clark MA, Thomas JM. Dr. Herbert Lumley Snow, MD, MRCS (1847-1930): the original champion of elective lymph node dissection in melanoma. Ann Surg Oncol 2004; 11:875-8.
- Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 2006; 355:1307-17.
- Fisher B, Fisher ER. Transmigration of lymph nodes by tumor cells. Science 1966; 152:1397-8.
- Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. The Lancet 1998; 351:793-6.
- Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage I melanoma of the limbs. N Engl J Med 1977; 297:627-30.
- Sim FH, Taylor WF, Pritchard DJ, Soule EH. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clin Proc 1986; 61:697-705.
- Balch C, Soong S, Ross M, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0-4.0 mm). Ann Surg Oncol 2000; 7:87-97.
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347:567-75.
- Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439-49.
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347:1662-9.
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998; 227:138-44.
- Lens MB, Dawes M, Goodacre T, Newton-Bishop JA. Elective lymph node dissection in patients with melanoma: systematic review and meta-analysis of randomized controlled trials. Arch Surg 2002; 137:458-61.
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014; 370:599-609.
- Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18:1232-42.
- Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 2007; 170:774-86.
- Cochran AJ, Pihl E, Wen DR, Hoon DS, Korn EL. Zoned immune suppression of lymph nodes draining malignant melanoma: histologic and immunohistologic studies. J Natl Cancer Inst 1987; 78:399-405.
- Cochran AJ, Morton DL, Stern S, et al. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod Pathol 2001; 14:604-8.
- Balch CM, Murad TM, Soong SJ, et al. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer 1979; 43:883-8.
- Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg 1999; 230:453-63; discussion 63-5.
- Balch CM, Cascinelli N, Sim FH. Elective lymph node dissection: results of prospective randomized surgical trials. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, eds. Cutaneous Melanoma. Saint Louis, MO: Quality Medical Publishing; 2003:379-95.
- Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol 2001; 8:101-8.
- Wright BE, Scheri RP, Ye X, et al. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg 2008; 143:892-9; discussion 9-900.
- Karakousis G, Gimotty PA, Bartlett EK, et al. Thin melanoma with nodal involvement: analysis of demographic, pathologic, and treatment factors with regard to prognosis. Ann Surg Oncol 2017; 24:952-9.
- Karakousis GC, Gimotty PA, Czerniecki BJ, et al. Regional nodal metastatic disease is the strongest predictor of survival in patients with thin vertical growth phase melanomas: a case for SLN staging biopsy in these patients. Ann Surg Oncol 2007; 14:1596-603.

