Cristina Carrera, MD, PhD
Dermatology Department
Melanoma Unit
Hospital Clínic de Barcelona
Universitat de Barcelona and
Centro de Investigación en
Red de Enfermedades Raras (CIBERER)
Instituto Carlos III, Barcelona, Spain
Ashfaq A. Marghoob, MD
Attending Physician
Dermatology Service
Department of Medicine
Memorial Sloan Kettering Cancer Center
Hauppauge, NY
Melanoma diagnosed during childhood and adolescence is extremely rare, accounting for 1 to 3 percent of all pediatric cancers and 1 to 4 percent of all melanomas. Incidence increases tenfold after puberty, with one case per 100,000 in those 15 to 19 years of age (updated SEER).1 Although this figure is extremely low, consultations regarding nevi account for 30 percent of visits to pediatric dermatologists.2 This is likely because new and changing nevi are common in young people.
The dynamic nature of nevi during youth partly explains why more than 2 million mole biopsies were performed in patients under age 19 between 2009 and 2013 in the U.S., though only 1,940 melanomas were diagnosed: That’s about 1,030 biopsies for every melanoma found.3 Unfortunately, even with this high biopsy ratio, pediatric melanoma diagnoses are delayed longer than 12 months in more than 60 percent of cases.4 Improved understanding of the clinical and dermoscopic morphology of these melanomas may allow timelier detection of these cancers while also eliminating many unnecessary nevus biopsies.
Three Categories of Childhood Melanoma
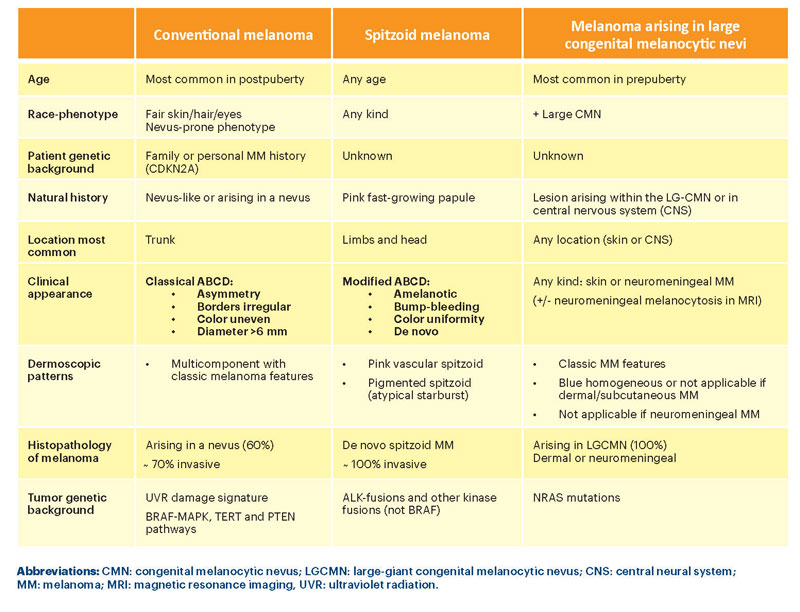
Based on clinicopathologic and molecular profiles, childhood melanomas can be classified into three broad categories:5 1) melanoma arising in the context of large congenital nevi, 2) spitzoid melanoma (SM) and 3) conventional melanoma (CM) (i.e., superficial spreading or nodular melanoma). We present key features and comparisons of each of these categories in Table 1.6
Even with the use of dermoscopy, early diagnosis of melanoma arising in large congenital melanocytic nevi remains challenging since most of these melanomas arise from deep within the nevi, often within the deep dermis. However, early diagnosis of CM and SM can be facilitated via recognition of these lesions’ clinical and dermoscopic features.7 CM in children display the classic melanoma-specific structures described for superficial spreading and nodular melanomas. Hence, these melanomas are relatively easy to detect via dermoscopy. Dermoscopy of CM often reveals an asymmetric multicomponent pattern, with the lesion displaying melanoma-specific structures such as atypical or negative network, pseudopods/streaks, shiny white structures, blue-white veil, peppering, polymorphous vessels, blue-black color and irregular blotches.
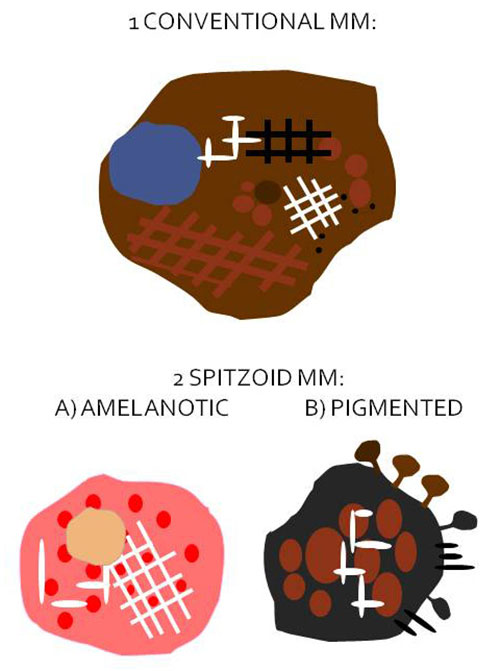
Figure 1. Schematics of the most frequent dermoscopic presentation of pediatric melanomas
1. Conventional melanoma: asymmetric pigmented lesion on the trunk, arising in a preexisting nevus showing multicomponent pattern (combination of network, globules and homogeneous areas) and/or the presence of any melanoma-specific features: atypical network, negative network, irregular globules/dots, blue-white veil, shiny white structures, atypical vessels or regression/peppering, in an otherwise symmetric nevus-like lesion (especially in early stages).
2. Spitzoid melanoma: de novo fast-growing lesion, mainly on limbs or head:
A) Amelanotic pattern: nonpigmented, symmetric papule or bump, showing atypical vessels (mainly dotted or milky-red areas), frequently with ulceration and shiny white structures or negative network.
B) Pigmented pattern (reed-like pattern): heavily pigmented papule or patch with the atypical starburst pattern: asymmetric peripheral streaks/pseudopods, shiny white structures, atypical black globules.
SM are a bit more challenging to diagnose. They usually present as fast-growing symmetric pink papules, often initially misdiagnosed as warts, dermatofibromas or vascular lesions. However, dermoscopy has helped to correctly identify these tumors. SM characteristically present with one of two patterns: 1) a starburst pattern in a heavily pigmented lesion or 2) a nonpigmented pattern with atypical vascular structures in a symmetric pink papule. Both patterns can also reveal shiny white structures on polarized dermoscopy. Thus, any symmetric lesion manifesting streaks, blue or black color, shiny white structures or polymorphous vascular structures should be viewed with suspicion and biopsied.
Even when we know the clinical and dermoscopic morphology, immuno-histochemistry and molecular signatures of spitzoid neoplasms, we cannot confidently classify some atypical spitzoid tumors as benign or malignant since their biologic potential remains unknown. In such cases, the most prudent management approach remains complete surgical removal of the lesion.8,9
In conclusion, awareness of the clinical and dermoscopic morphology of pediatric melanoma can help avoid delays in diagnosis and reduce the high burden of excisions of banal nevi in children.
References:
- Austin MT, Xing Y, Hayes-Jordan AA, et al. Melanoma incidence rises for children and adolescents: an epidemiologic review of pediatric melanoma in the United States. J Pediatr Surg 2013; 48(11):2207-2213. doi:10.1016/j.jpedsurg.2013.06.002. https://seer.cancer.gov/csr/1975_2015/results_merged/sect_16_melanoma_skin.pdf.
- Aguilera P, Puig S, Guilabert A, et al. Prevalence study of nevi in children from Barcelona. Dermoscopy, constitutional and environmental factors. Dermatology 2009; 218(3):203-214. doi:10.1159/000183179.
- Oliveria SA, Selvam N, Mehregan D, et al. Biopsies of nevi in children and adolescents in the United States, 2009 through 2013. JAMA Dermatol 2015; 151(4):447-448. doi:10.1001/jamadermatol.2014.
- Mitkov M, Chrest M, Diehl NN, et al. Pediatric melanomas often mimic benign skin lesions: a retrospective study. J Am Acad Dermatol 2016; 75(4):706-711.e4. doi:10.1016/j.jaad.2016.05.015.
- Lu C, Zhang J, Nagahawatte P, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol 2015; 135(3):816-823. doi:10.1038/jid.2014.425.
- Cordoro KM, Gupta D, Frieden IJ, et al. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol 2013; 68(6):913-925. doi:10.1016/j.jaad.2012.12.953.
- Carrera C, Scope A, Dusza SW, et al. Clinical and dermoscopic characterization of pediatric and adolescent melanomas: Multicenter study of 52 cases. J Am Acad Dermatol 2018; 78(2):278-288. doi: 10.1016/j.jaad.2017.09.065. Epub 2017 Oct 9.
- Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol 2015; 72(1):47-53. doi:10.1016/j.jaad.2014.09.037.
- Moscarella E, Lallas A, Kyrgidis A, et al. Clinical and dermoscopic features of atypical Spitz tumors: a multicenter, retrospective, case-control study. J Am Acad Dermatol 2015; 73(5):777-784. doi:10.1016/j.jaad.2015.08.018.