Vernon K. Sondak, MD
Department of Cutaneous Oncology
Jane L. Messina, MD
Department of Cutaneous Oncology
Department of Anatomic Pathology
Damon Reed, MD
Department of Cutaneous Oncology
Department of Sarcoma and the Adolescent and Young Adult Oncology Program
Moffitt Cancer Center, Tampa, Florida
Melanoma in childhood has gained attention as the deadliest form of pediatric skin cancer. Childhood melanoma largely takes two distinct forms: the rare melanoma that arises in giant congenital nevi and the more common sporadic form. Most pediatricians now realize the importance of prompt evaluation and, when appropriate, excision of giant congenital nevi. But the sporadic form of childhood melanoma is roughly 10 times more common, and the use of tanning beds has undoubtedly contributed to the increasing incidence of this disease among young people.1,2,3 This has prompted important legislative efforts aimed at curbing tanning bed use in the adolescent population. To date, 42 states and the District of Columbia have either restricted adolescent tanning bed usage or banned it entirely.4
Diagnostic Challenges
Increased awareness of the pediatric occurrence of a cancer previously thought to be almost exclusively entrenched in the adult population has led to greater scrutiny of children’s pigmented lesions by parents and primary care practitioners alike. This has led to increased referral of children to dermatologists, generated greater numbers of skin lesion biopsies and revealed that a significant number of melanocytic neoplasms in children are diagnostically challenging, both clinically and histologically.
These neoplasms are known by a variety of terms, e.g., melanocytic tumors of uncertain malignant potential (MELTUMP) and atypical melanocytic proliferation (AMP).5 Many bear a resemblance to the benign skin lesions first described by Sophie Spitz and are therefore termed atypical Spitz tumors (AST) or spitzoid tumors of undetermined malignant potential (STUMP). As pathologists seek to determine whether these histologically ambiguous lesions are benign or represent potentially deadly melanomas, they employ a variety of molecular tests aimed at better defining their risk. These include fluorescence in situ hybridization (FISH), comparative genomic hybridization (CGH) and, more recently, evaluation for TERT promoter mutations.6 Despite these advances in molecular evaluation, a significant number of cases remain in diagnostic uncertainty after thorough workup. Proper management along the spectrum between benign lesion and melanoma poses great challenges for the physician, both in communicating the risk to an anxious family and in determining adequate but not overzealous treatment and follow-up.
The Keys to Proper Treatment
It is axiomatic throughout oncology (and throughout medicine in general) that proper treatment begins with a proper diagnosis; diagnostic uncertainty not only dramatically elevates patient and family concerns and anxiety levels but starts the therapeutic process off on the wrong foot. Along with the challenge of accurate diagnostic classification, pathologists also must accurately convey their assessments to the treating clinicians. Hence, optimal care of children with atypical or frankly malignant melanocytic neoplasms requires multidisciplinary efforts and accurate communication of test results and inferred risk, not only within the medical team but for patients and their families.
The Moffitt Five-Point Scale for Reporting Melanocytic Neoplasia
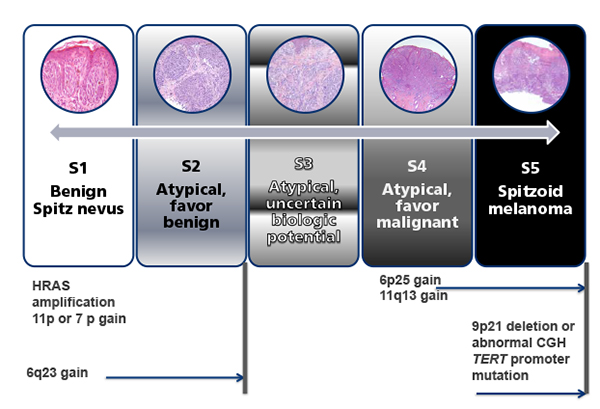
We have found that a five-point scale modeled in part on the five-point BI‑RADS classification (Breast Imaging Reporting and Data System), developed by radiologists to convey risk of mammographic findings, can be an extremely useful tool for communication from pathologist to clinician, and in turn from clinician to the family and patient.7 Our team adapted this alphanumeric system to integrate the findings of all available histologic, immunohistochemical and molecular test results and to communicate the histologic class and imputed risk of malignancy to clinicians and family (Figure 1).
Figure 1. The spectrum of melanocytic neoplasia in spitzoid lesions on the Moffitt five-point scale, ranging from a benign Spitz nevus (category 1) through varying degrees of atypia (categories 2 through 4) all the way to spitzoid melanoma (category 5).
Genetic aberrations as assessed by fluorescence in situ hybridization and/or comparative genomic hybridization can be particularly helpful in classifying atypical lesions as either “favor benign” (category 2) or “favor malignant” (category 4), but even with all available histologic and ancillary information, some lesions remain difficult or impossible to categorize (category 3) as either “favor benign” or “favor malignant.” The goal of the schema is to ensure accurate communication of the pathologist’s assessment to the clinician. Courtesy of Jane Messina, MD; modified from Sreeraman Kumar R, Messina JL, Reed D, Navid F, Sondak VK. Pediatric melanoma and atypical melanocytic neoplasms. Canc Treat Res 2016; 167:331–369.
First, the histologic class is assigned a letter describing its major feature. As most of these lesions resemble Spitz nevi, the most common prefix is S; others include B (blue nevus-like), C (congenital) and N (nevoid). Next, a number from 1 to 5 computing the assessed determination and expressing the degree of confidence in the benign or malignant nature of the lesion is assigned. Lesions determined to be unequivocally benign are assigned a score of 1, while clearly malignant lesions are scored a 5. Lesions with some features felt to be benign are assigned a 2, while those with serious abnormalities favored to be malignant are rated 4. Lesions are scored 3 when the balance of testing cannot determine their malignant potential. Note that this classification schema does not specifically assign histologic findings to one category or another, and two pathologists might view the same lesion quite differently. The goal of the schema is not to achieve diagnostic consensus but to ensure accurate communication of the pathologist’s assessment to the clinician.
For example, a lesion with the histology of an atypical Spitz tumor that demonstrates heterozygous loss of 9p21 on FISH would be described as an S4, as studies with limited follow-up have shown that these lesions have a propensity to metastasize to regional lymph nodes but rarely disseminate further. Patients in each diagnostic category receive customized surgical treatment aimed at reducing risk of recurrence, regional nodal involvement and dissemination. At our institution, we treat S1 and S2 lesions with complete excision. We treat S3 and S4 lesions surgically to account for the “worst-case scenario,” as if they were melanomas of comparable thickness. This includes wide excision and, when the lesion is ≥0.8 mm thick and/or ulcerated (T1b in the AJCC 8th edition staging system), sentinel lymph node biopsy; S5 is treated using standard guidelines for melanoma.9
Findings in the excision specimen or sentinel node can sometimes add to and modify the findings of the original biopsy and may result in reclassification of an S3 or S4 lesion to an unequivocally malignant S5. It is important to note that nearly all of our knowledge about “borderline lesions” and pediatric melanoma comes from retrospective reviews and registries.5,10,11,12,13 Lesions considered to have been “atypical” in one series may later recur and be included in a different series as melanoma. While it is appropriate that each piece of clinical and pathological information be incorporated into diagnostic and therapeutic decision-making, having a clear record of what the lesion was thought to be at initial diagnosis and after definitive surgery (akin to the concepts of “clinical staging” and “pathological staging” embraced by the AJCC melanoma staging system) will go a long way to shed more light on the natural history of lesions recognized as atypical but not unequivocally malignant at initial diagnosis.
Surgical Management: Similar to Treatment in Adults
While the diagnostically challenging subset of pediatric melanocytic lesions garners much attention in the literature, the majority of melanocytic tumors removed in older adolescents are clinically and pathologically identical to adult melanoma. In general, surgical management of pediatric melanoma is nearly the same as in adults, with the distinction that we rarely if ever employ excision margins greater than 1 cm for children 14 or younger.9 Local recurrences of pediatric melanomas excised with a 1-cm margin have been essentially nonexistent in our experience. Sentinel lymph node biopsy is widely employed as a diagnostic and staging adjunct, but (as has recently become standard in adults) radical lymphadenectomy is often omitted for patients with positive sentinel lymph nodes. In contrast, clinically detected nodal involvement is routinely managed with radical lymphadenectomy, and we have now treated several pediatric patients successfully with neoadjuvant molecularly targeted therapy prior to lymphadenectomy, a strategy that is being employed increasingly in adults with BRAF-mutant clinical stage III melanoma.
Adjuvant Therapy
Adjuvant therapy for adult stage III melanoma has changed dramatically in the past five years. Interferon α-2b [Intron A®], once the only approved adjuvant option, has essentially been replaced by adjuvant use of immune checkpoint inhibitors (initially ipilimumab [Yervoy®] but now anti-PD1 antibodies, with nivolumab [Opdivo®] FDA-approved for this indication), as well as adjuvant use of combination dabrafenib [Tafinlar®] and trametinib [Mekinist®], a newly FDA-approved option for BRAF-mutant stage III melanoma. For pediatric patients, interferon α-2b, including the pegylated form, has proven better tolerated than in adults, and has been widely employed for children with sentinel node-positive or clinical stage III melanoma after surgery.14
Virtually no pediatric experience with adjuvant use of either ipilimumab or anti-PD1 antibodies has been reported. While adjuvant anti-PD1 monotherapy is generally quite well tolerated in adults, long-term endocrine and cardiac toxicities are recognized and would be potentially catastrophic developments in children.15 Moreover, troublesome joint-related problems, likely various forms of autoimmune arthritis, have also emerged as late sequelae of immunotherapy treatment and could also disproportionately impact on a younger population.16 Since BRAF mutations are common in pediatric melanoma, and since experience with BRAF and MEK inhibition in children has largely been favorable, we have recently considered combination dabrafenib-trametinib to be a more attractive option for adjuvant therapy of stage III pediatric melanoma when a BRAF mutation is present. BRAF mutation testing by immunohistochemistry and pyrosequencing or next-generation sequencing is now routinely conducted on all stage III melanoma cases at our institution.
Treating Stage IV Melanoma
Fortunately, stage IV melanoma is rare in pediatric patients. However, many cases of stage IV melanoma in young adults originated from primary tumors that arose before age 18;11 hence, there is more “metastatic pediatric melanoma” than many oncologists realize. Despite the explosion of new treatment options for unresectable metastatic melanoma in adults (at last count, at least 11 drugs or combinations have received FDA approval for this indication since 2011), only one drug — ipilimumab — has received FDA approval for treatment of metastatic melanoma in children. Even this approval was not based on extensive experience in the pediatric population.17
Nevertheless, the drugs commonly used in adult stage IV melanoma represent the best option for those rare pediatric-aged patients with unresectable metastatic melanoma. Concerns over late toxicity in childhood melanoma survivors are a factor in unresectable metastatic melanoma, but in contrast to the adjuvant setting, these concerns take a back seat to the need for effective treatment of an immediately life-threatening condition. Hence, immunotherapy tends to be our first-line treatment option for stage IV melanoma in children just as in adults, using either single-agent anti-PD1 therapy (nivolumab or pembrolizumab [Keytruda®]) or combination ipilimumab plus nivolumab. As in adults, decision-making about single-agent versus combination immunotherapy involves complex calculations weighing the greater risk of toxicity against the higher response rate and progression-free survival with combination treatment. We generally prefer to use single-agent anti-PD1 therapy unless the disease is symptomatic, or when the tumor burden is very high even if asymptomatic, or when brain metastases are present in conjunction with extra-CNS disease.
BRAF-mutant unresectable metastatic melanoma that is refractory to immunotherapy is treated with combination BRAF-MEK inhibition. There are now three FDA-approved combinations for adult use (dabrafenib+trametinib, vemurafenib [Zelboraf®]+cobimetinib [Cotellic®] and encorafenib [BraftoviTM] +binimetinib [Mektovi®]), with little to indicate whether one combination would be better suited to pediatric use than another.
Cytotoxic chemotherapy has almost entirely been replaced as a treatment for metastatic melanoma by these various new drugs.
Conclusions
While the treatment strategies developed in adults have proven highly efficacious in most cases when adapted to pediatric patients, there is clearly a need for more prospective testing of therapeutic strategies in the pediatric population. Cooperative group, multicenter and even multinational studies will be required for adequate testing, given the rarity of advanced melanoma in childhood. Moreover, centers with extensive experience in treating young adults should be encouraged to reassess their experience in those cases where the original melanoma was diagnosed in childhood. This will help in seeking further insights into optimal treatment approaches in both adjuvant and metastatic settings.
For the foreseeable future, we urge clinicians to refer all patients with pediatric melanoma or atypical melanocytic proliferations to clinical centers that have dedicated teams for the pathological evaluation and clinical management of these challenging but highly rewarding cases. We have found that excellent communication between family, pathologist, radiologist, surgical team, oncologist and referring physician is critical for the creation and implementation of the optimal plan. Educational efforts directed at pediatricians, family medicine providers and dermatologists would help further raise awareness of pediatric melanoma and the challenges faced by patients, families and providers when confronted with a suspicious pigmented lesion on a child’s skin.
References
- Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the Surveillance, Epidemiology and End Results database. J Clin Oncol 2005; 23(21):4735-4741.
- Austin MT, Xing Y, Hayes-Jordan AA, et al. Melanoma incidence rises for children and adolescents: an epidemiologic review of pediatric melanoma in the United States. J Pediatr Surg 2013; 48(11):2207-2213.
- Wong JR, Harris JK, Rodriguez-Galindo C, Johnson KJ. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics 2013; 131(5):846-854.
- Madigan LM, Lim HW. Tanning beds: Impact on health, and recent regulations. Clin Dermatol 2016; 34(5):640-648.
- Mills OL, Marzban S, Zager JS, et al. Sentinel node biopsy in atypical melanocytic neoplasms in childhood: a single institution experience in 24 patients. J Cutan Pathol 2012; 39(3):331-336.
- Bahrami A, Barnhill RL. Pathology and genomics of pediatric melanoma: a critical reexamination and new insights. Pediatr Blood Cancer 2018; 65(2).
- Sondak VK, Reed D, Messina JL. A comprehensive approach to pediatric atypical melanocytic neoplasms with comment on the role of sentinel lymph node biopsy. Crit Rev Oncog 2016; 21(1-2):25-36.
- Sreeraman KR, Messina JL, Reed D, et al. Pediatric melanoma and atypical melanocytic neoplasms. Cancer Treat Res 2016; 167:331-369.
- Reed D, Kudchadkar R, Zager JS, et al. Controversies in the evaluation and management of atypical melanocytic proliferations in children, adolescents, and young adults. J Natl Compr Canc Netw 2013; 11(6):679-686.
- Bailey KM, Durham AB, Zhao L, et al. Pediatric melanoma and aggressive Spitz tumors: a retrospective diagnostic, exposure and outcome analysis. Transl Pediatr 2018; 7(3):203-210.
- Han D, Zager JS, Han G, et al. The unique clinical characteristics of melanoma diagnosed in children. Ann Surg Oncol 2012; 19(12):3888-3895.
- Lange JR, Palis BE, Chang DC, et al. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J Clin Oncol 2007; 25(11):1363-1368.
- Averbook BJ, Lee SJ, Delman KA, et al. Pediatric melanoma: analysis of an international registry. Cancer 2013; 119(22):4012-4019.
- Navid F, Herzog CE, Sandoval J, et al. Feasibility of pegylated interferon in children and young adults with resected high-risk melanoma. Pediatr Blood Cancer 2016; 63(7):1207-1213.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36(17):1714-1768.
- Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol 2018 Aug 31. doi: 10.1038/s41584-018-0074-9 [published online ahead of print].
- Geoerger B, Bergeron C, Gore L, et al. Phase II study of ipilimumab in adolescents with unresectable stage III or IV malignant melanoma. Eur J Cancer 2017; 86:358-363.