Alon Scope, MD
Medical Screening Institute
Sheba Medical Center and
Sackler School of Medicine
Tel Aviv University, Israel
Dermatology Service, Department of Medicine,
Memorial Sloan Kettering Cancer Center, New York City
Cutaneous melanoma is a potentially lethal disease that offers a unique chance for early detection and cure like no other cancer. The late Bernard Ackerman, MD, spelled out the goal of early melanoma detection succinctly in his aptly titled 1985 paper, “No One Should Die of Malignant Melanoma.”1
To achieve early detection, clinicians have proposed multiple clinical strategies, including the ABCDE early recognition criteria [A (asymmetry), B (border irregularity), C (color variegation), D (diameter >6 mm), E (“evolving”)2] and the “ugly duckling” sign. The latter highlights that melanoma can be an outlier lesion, differing in size, color and pattern from the patient’s benign nevi, which often appear similar to one another.3
Physicians have also improved diagnostic accuracy by incorporating imaging technologies in clinical practice. Dermoscopy enables evaluation, at higher magnification, of subsurface patterns and structures that are not visible to the naked eye; dermoscopic digital monitoring allows short-term follow-up of the dermoscopic pattern of melanocytic lesions for stability versus change over time. Total-body photography also assists in long-term monitoring, whereby a melanoma can be detected as a new or changing lesion among the patient’s host of stable benign nevi.
Despite all these diagnostic techniques, some melanomas elude diagnosis; not only very early or small-diameter melanomas, but also nodular, amelanotic and nevoid melanomas, as well as those arising on sun-damaged skin. Difficult-to-diagnose melanomas need to be differentiated from the patient’s benign nevi. In such scenarios, physicians may glean subtle diagnostic clues from the clinical context, taking into account different patient-related factors.4
For the most part, a patient’s nevi abide by “rules” — their morphology and biological behavior depend on the patient’s demographic, phenotypic, genetic and environmental factors. If we decipher these rules that govern the morphology of nevi, we may be able to detect an otherwise difficult-to-diagnose melanoma that is breaking these rules.
The Importance of Childhood Nevi
Melanocytic nevi are a strong phenotypic marker of cutaneous melanoma risk.5 Nevi frequently develop in the first two decades of life, making this a prime period for studying when, where and how nevi should appear. The Study of Nevi in Children (SONIC), a population-based study centered in Framingham, Massachusetts, has documented the morphology of nevi and their evolution over time during childhood and adolescence. We accrued participants at ages 11 to 14 from all schools in the Framingham district and followed a subset of their nevi, using clinical and dermoscopic imaging, until age 17. By administering questionnaires to participants and their parents and conducting on-site skin examinations, we collected demographic, clinical, phenotypic and environmental exposure data.6
Here, we offer examples of nevus rules that we established from the SONIC project and from other studies of nevi, and the implications for detecting a melanoma that is breaking these rules.7 Obviously, there are exceptions; not every lesion that deviates from the expected pattern is an obligatory melanoma. Nonetheless, a deviant lesion should be approached judiciously to exclude melanoma.
Rule: The pattern of nevi depends on the patient’s age
Multiple studies have shown a major shift in the predominant dermoscopic pattern of nevi from childhood to adulthood.8 The most frequent dermoscopic nevus pattern in children is the globular type (Figure 1A), and the most frequent pattern in adults is the reticular type (Figure 1B). In the SONIC cohort, at age 11, 34 percent of back nevi were globular, while only 11 percent were reticular.9 During follow-up from age 11 to age 17, we observed a gradual decline in the percentage of globular nevi and an increase in that of reticular nevi. This pattern shift was not due to change within individual nevi, but the appearance of more nevi with reticular patterns; at age 17, of new back nevi, 44 percent were reticular and only 16 percent were globular (unpublished data). This globular to reticular pattern shift continues into adulthood; in patients in their fourth decade, 61 percent of trunk nevi were reticular, while only 20 percent were globular.8
Another dermoscopic pattern of nevi that is highly age-dependent is the “peripheral rim of globules” (PRG) pattern (Figure 1E); this pattern signifies a growing nevus.10 When the nevus ceases to grow, the peripheral rim of globules disappears and the nevus assumes one of the other patterns (e.g., reticular pattern). In the second decade, PRG nevi were observed in 3 percent of back nevi among SONIC participants10 and in 5 percent of trunk nevi among adolescent patients visiting pigmented lesion clinics.8 Notably, the frequency of PRG pattern rapidly declines with age — these lesions are seen in 1.5 percent of trunk nevi in the fourth decade, and in <1 percent of patients >50 years old.8
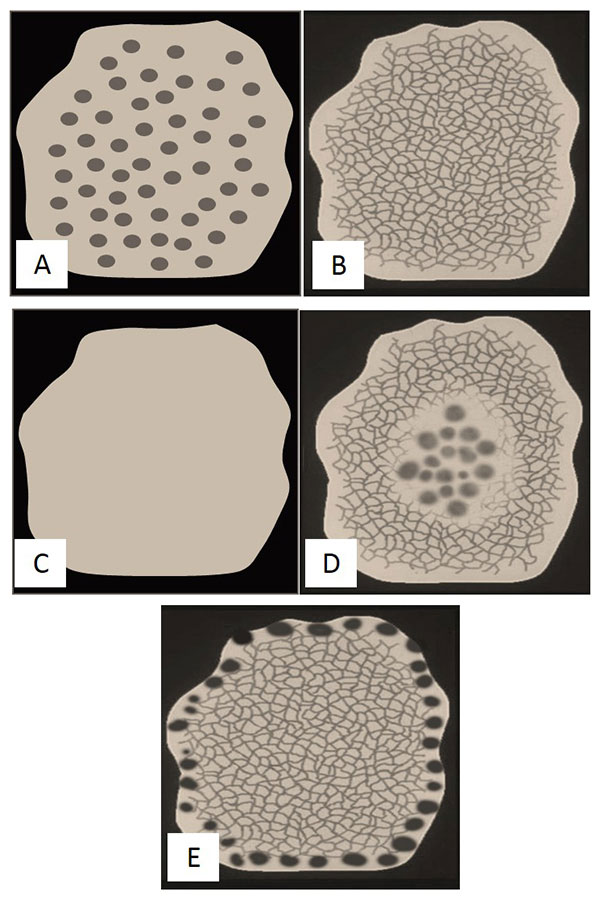
Figure 1. Common dermoscopic patterns of nevi.
(A) Globular pattern, (B) Reticular pattern, (C) Homogeneous/Structureless pattern,
(D) Complex (reticular-globular) pattern, (E) Growing nevus with peripheral rim of globules (PRG) pattern.
Significance for melanoma diagnosis
A new nevus with a globular dermoscopic pattern in an elderly patient is uncommon. Notably, a recently described subtype of melanoma termed “nested melanoma of the elderly”11 can be recognized as a lesion with a diffuse globular pattern appearing in a patient at the “wrong age.” Furthermore, melanomas that display a predominantly globular dermoscopic pattern have been shown to grow faster than melanomas that show a predominantly reticular pattern.12 Finally, pigmented lesions that show a PRG pattern in a patient >50 years old should raise suspicion for melanoma.
Rule: The pattern of nevi depends on body site
In SONIC, we observed that the size and dermoscopic pattern of nevi depends on the anatomic site of the nevi.6,13 First, nevi on the upper trunk tend to be larger in diameter and show a globular dermoscopic pattern (Figure 2A), whereas nevi on the lower trunk and extremities become increasingly smaller and tend to be more frequently reticular in dermoscopic pattern (Figure 2B). Second, nevi with a PRG pattern are very rare on the legs. Third, nevi with a complex dermoscopic pattern (Figure 1D), showing both globular and reticular components, occur more frequently on the trunk than on the extremities (Figure 2C).
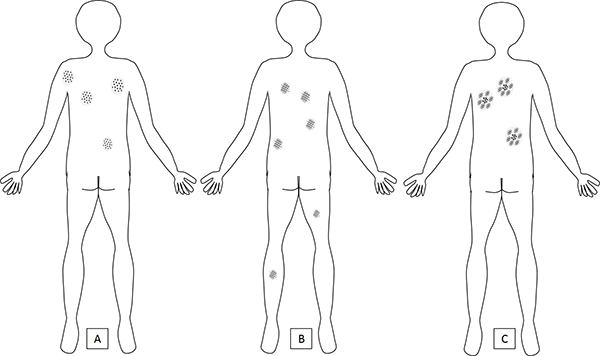
Figure 2. Dermoscopic pattern of nevi by body site.
A) Globular nevi are more prevalent on the torso, particularly on the upper back, where they tend to be larger and more numerous than globular nevi on the lower back. On the lower extremities, globular nevi are uncommon. Globular nevi are the dominant pattern in children.
B) Reticular nevi are the dominant pattern in adults and can be seen both on the torso and on the extremities. They are mostly smaller in diameter than globular or complex nevi.
C) Complex pattern nevi are seen more frequently in adults with a high nevus count that includes large nevi (the so-called “atypical mole phenotype”). Complex nevi are more common on the torso and infrequent on the lower extremities.
Some exceptions exist; for example, a congenital (“birthmark”) nevus that is located on the lower extremities may be the patient’s largest mole; patients with the so-called “atypical nevus phenotype,” who have a high nevus count and large nevi (>5 mm in diameter), may show nevi with a complex pattern on the extremities as well as on the trunk.
Significance for melanoma diagnosis
(Figures 3A-3B): An example of a suspect lesion breaking the anatomic site rules would be an acquired, singular pigmented lesion on the lower extremities of an adult that is larger in diameter than the patient’s trunk nevi, and/or shows a globular, complex or PRG dermoscopic pattern.
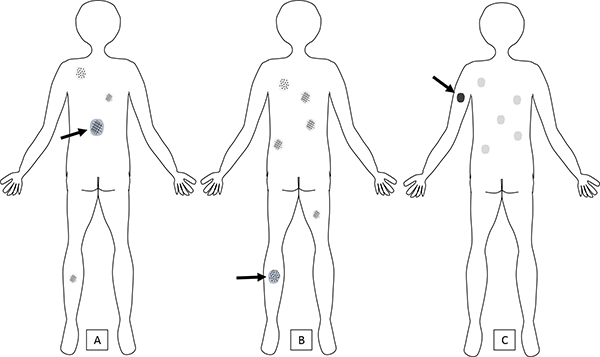
Figure 3. Melanomas that “break the rules.”
A) The lesion with reticular pattern on the lower back (arrow) is larger than the patient’s other upper back nevi, including the globular nevi. This lesion is breaking the size gradient — upper trunk nevi >lower trunk nevi and globular >reticular; if it is an acquired lesion in an adult, it should raise suspicion for melanoma. At times, melanomas arising on sun-damaged skin may present in this manner.
B) A globular lesion on the leg (arrow) of an older individual without a history of long-standing stability is suspicious for melanoma. New globular nevi are more likely to be observed in children and adolescents, and their frequency decreases in adulthood. Nevi on the lower extremities are more likely to be reticular than globular.
C) This patient has a light pigmentary phenotype with multiple light-colored (fair to light brown) nevi. The melanoma (arrow) is an acquired lesion that is too dark for the patient’s skin type.
Rule: The pattern of nevi depends on the patient’s skin color
In SONIC and in other studies, a predictable association has been observed between a patient’s skin color and the dermoscopic pattern and size of nevi.6,14 Participants with darker skin tend to have nevi that are smaller in diameter and reticular in dermoscopic pattern; in contrast, participants with fair skin more frequently harbor larger nevi with a globular or complex dermoscopic pattern. Another subset of nevi are those that lack a specific dermoscopic pattern (termed “structureless” or “homogeneous” nevi, Figure 1C): Structureless, light-colored nevi are more frequent among fair-skinned participants. Skin color has been associated with germline variations in the gene for melanocortin-1 receptor (MC1R); indeed, individuals who harbor a mutation in MC1R, which is associated with fair phenotype and red hair color, show structureless nevi more frequently, whereas those with MC1R variants associated with darker skin type tend to show nevi with a reticular pattern.15
Significance for melanoma diagnosis
An outlier, darkly pigmented lesion in an individual with a fair-skinned phenotype should raise suspicion for melanoma (Figure 3C). In addition, individuals with very fair skin, particularly those with an MC1R mutation associated with red hair color, may pose a particular challenge for melanoma diagnosis.16 While their nevi tend to appear lightly pigmented and structureless under dermoscopy, a melanoma in such a patient may also lack pigment (termed “amelanotic melanoma”) and display a structureless dermoscopic pattern. Hence, these very fair patients may benefit from closer surveillance using total-body photography to detect melanoma as a new or changing lesion.
Rule: Change in nevi is frequent in childhood
Among SONIC participants, we frequently saw newly appearing, changing and disappearing nevi.9,17 Between ages 11 and 14, 75 percent of participants developed new nevi, while 28 percent had at least one nevus that disappeared. Of the new nevi detected at age 14, only half were stable over the next three years, while 43 percent increased in size and 5 percent disappeared. Disappearing nevi often gradually faded in color and/or shrank in diameter. Children with the highest baseline nevus counts were also those with the highest nevus volatility, i.e., with a proclivity for new, changing and disappearing nevi. We also observed that among children with many nevi, a history of sunburn could be associated with a greater increase in number of nevi.15
Significance for melanoma diagnosis
Use of total-body photography in children and adolescents to identify suspicious lesions based on change is likely to have poor specificity for the diagnosis of melanoma. The infrequency of melanoma, taken together with the high nevus volatility in the first two decades of life, make the use of total-body photography a less effective strategy for melanoma detection than in adults.
Rule: Melanoma risk is related to the patient’s nevus phenotype
Having numerous nevi and large nevi (>5 mm in diameter) is associated with a high-risk phenotype for developing melanoma.5 In SONIC, we found that children harboring more nevi than their peers at age 14 were those most likely to develop a high-risk nevus phenotype at age 17.18 Individuals whose nevi showed greater variability in dermoscopic patterns (i.e., co-occurrence of nevi with globular, reticular and complex patterns) were also more likely to develop a high-risk nevus phenotype.18
Across SONIC studies, we have shown that subsets of nevi that can be recognized by their dermoscopic patterns (e.g., reticular nevi and globular nevi) are biologically distinct. As previously noted, globular and reticular nevi differ in their associations with the patient’s age and skin color, as well as in their predilection for different body sites.6 Furthermore, we have shown in a convenience sample from adult patients that these subsets of nevi also differ in their genetic profile — globular nevi are associated with BRAF V600E mutation, which is also frequently observed in melanoma, whereas reticular nevi are mostly BRAF-mutation-negative.19
Relevance for melanoma diagnosis
Interestingly, in a pilot case-control study, we found that patients with melanoma more frequently harbored nevi with a complex (reticular-globular) pattern (Figure 1D), compared to patients without melanoma. We are currently expanding this study, to better characterize nevus phenotypes that herald a heightened melanoma risk.
Conclusions
We have gained important insights about the expected morphology and host associations of childhood nevi from SONIC and other studies. A comprehensive understanding of the rules by which nevi abide may help in the early detection of melanomas that break these rules. In addition, to inform melanoma prevention and screening efforts, we need to better stratify patients based on their melanoma risk. A thorough understanding of nevogenesis and of nevus phenotypes may improve our ability to predict patients’ melanoma risk.
References
- Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol 1985; 12:115-6.
- Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004; 292:2771-6.
- Scope A, Dusza SW, Halpern AC, et al. The “ugly duckling” sign: agreement between observers. Arch Dermatol 2008; 144:58-64.
- Zalaudek I, Docimo G, Argenziano G. Using dermoscopic criteria and patient-related factors for the management of pigmented melanocytic nevi. Arch Dermatol 2009; 145:816-26.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer 2005; 41:28-44.
- Scope A, Marghoob AA, Dusza SW, et al. Dermoscopic patterns of naevi in fifth grade children of the Framingham school system. Br J Dermatol 2008; 158:1041-9.
- Scope A, Marchetti MA, Marghoob AA, et al. The study of nevi in children: Principles learned and implications for melanoma diagnosis. J Am Acad Dermatol 2016; 75:813-823.
- Zalaudek I, Schmid K, Marghoob AA, et al. Frequency of dermoscopic nevus subtypes by age and body site: a cross-sectional study. Arch Dermatol 2011; 147:663-670.
- Scope A, Dusza SW, Marghoob AA, et al. Clinical and dermoscopic stability and volatility of melanocytic nevi in a population-based cohort of children in Framingham school system. J Invest Dermatol 2011; 131:1615-21.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol 2015; 151:1338-1345.
- Longo C, Zalaudek I, Piana S, et al. Dermoscopy and confocal microscopy of nested melanoma of the elderly: recognizing a newly defined entity. JAMA Dermatol 2013; 149:941-945.
- Beer J, Xu L, Tschandl P, Kittler H. Growth rate of melanoma in vivo and correlation with dermatoscopic and dermatopathologic findings. Dermatol Pract Concept 2011; 1:59-67.
- Fonseca M, Marchetti MA, Chung E, et al. Cross-sectional analysis of the dermoscopic patterns and structures of melanocytic naevi on the back and legs of adolescents. Br J Dermatol 2015; 173:1486-1493.
- Zalaudek I, Argenziano G, Mordente I, et al. Nevus type in dermoscopy is related to skin type in white persons. Arch Dermatol 2007; 143:351-356.
- Quint KD, van der Rhee JI, Gruis NA, et al. Melanocortin 1 receptor (MC1R) variants in high melanoma risk patients are associated with specific dermoscopic ABCD features. Acta Derm Venereol 2012; 92:587-592.
- Cuéllar F, Puig S, Kolm I, et al. Dermoscopic features of melanomas associated with MC1R variants in Spanish CDKN2A mutation carriers. Br J Dermatol 2009; 160:48-53.
- Oliveria SA, Scope A, Satagopan JM, et al. Factors associated with nevus volatility in early adolescence. J Invest Dermatol 2014; 134:2469-2471.
- Xu H, Marchetti MA, Dusza SW, et al. Factors in early adolescence associated with a mole-prone phenotype in late adolescence. JAMA Dermatol 2017; 153:990-998.
- Marchetti MA, Kiuru MH, Busam KJ, et al. Melanocytic naevi with globular and reticular dermoscopic patterns display distinct BRAF V600E expression profiles and histopathological patterns. Br J Dermatol 2014; 171:1060-5.
The author gratefully acknowledges the grant support of the National Institutes of Health for the SONIC study.

